1. Background
Breast carcinoma (BC) is the most prevalent form of cancer in women and is classified into various subtypes based on the expression levels of receptors for estrogen (ER), progesterone (PR), and human epidermal growth factor 2 (HER2) (1). Advanced BC presents significant treatment challenges, with therapeutic options varying depending on the specific subtype (2). Recent research highlights the critical role of modulating apoptotic signaling pathways to reduce tumor burden in the fight against BC (3). Apoptosis, commonly referred to as programmed cell death, occurs through two main signaling pathways: Intrinsic and extrinsic. The intrinsic pathway is triggered by intracellular signals that lead to the release of proteins from the mitochondrial intermembrane space, while the extrinsic pathway is initiated by the binding of extracellular ligands to death receptors on the cell surface (4). Breast carcinoma cells frequently acquire resistance to apoptosis through multiple mechanisms, including the overexpression of anti-apoptotic molecules such as Bcl-2 and the downregulation of pro-apoptotic factors like Bax. These alterations enable cancer cells to evade apoptosis, thereby promoting tumor growth and enhancing resistance to conventional therapies. Targeting apoptotic pathways presents a promising strategy for cancer treatment, with the goal of restoring the balance between pro-apoptotic and anti-apoptotic signals, consequently sensitizing cancer cells to apoptosis induced by treatment (5).
Mesenchymal stem cells (MSCs) have garnered attention as potential therapeutic agents due to their multipotency, immune privilege, and immunomodulatory properties. However, the literature presents conflicting evidence regarding the effects of MSCs on cancer growth, which can vary depending on the cancer type and MSC source (6). Wharton's jelly-derived mesenchymal stem cell secretomes have shown promise in cancer therapy due to their ability to express elevated levels of apoptosis-related genes and release soluble factors. These properties make from Wharton's jelly mesenchymal stem cells (WJ-MSC) secretomes suitable candidates for allogeneic cell therapy, offering superior expansion capacity and immune privilege (7).
2. Objectives
Despite the potential of WJ-MSC secretomes, there are gaps in understanding their specific mechanisms of action, particularly regarding their impact on apoptosis in HER2-positive breast cancer cells. Conflicting results from previous studies highlight the need for further investigation (8-10). This study aims to address these gaps by examining the anti-proliferative and pro-apoptotic effects of WJ-MSC secretomes on the HER2-positive human mammary epithelial cell line SKBR3, as well as on a normal cell line (MCF 10A). The originality of this study lies in its comprehensive examination of the distinct anti-cancer properties of WJ-MSC secretomes, particularly their effect on HER2-positive breast cancer cells. By elucidating these mechanisms, this research seeks to provide insights into the potential therapeutic applications and mechanisms of action of WJ-MSC secretomes, with a specific focus on apoptotic pathways and the expression of related genes.
3. Methods
3.1. Isolation of Wharton's Jelly Mesenchymal Stem Cells
Following a standardized protocol approved by the Abadan University Ethics Committee (approval number: IR.ABADANUMS.REC.1399.142), we isolated WJ-MSCs based on a previous study conducted by our team. Human umbilical cords were collected from full-term newborns delivered by cesarean section, with written informed consent obtained from the parents. Only cords from healthy infants were selected to minimize the risk of microbial contamination. The umbilical cords were thoroughly cleaned with PBS, the vessels were removed, and the cords were cut into 2 cm segments. Wharton’s jelly tissue (4 - 5 mm pieces) was carefully dissected and individually placed into T25 flasks. After 5 - 10 minutes, 5 mL of complete DMEM medium containing 1% L-glutamine, 10% fetal bovine serum (FBS), and 1% penicillin-streptomycin solution was added to each flask. The flasks were then incubated at 37°C in a 5% CO2 atmosphere.
Once the cells reached 80% confluence, they were subcultured. At passage 3, the cells were characterized for mesenchymal stem cell markers by incubating them with antibodies against CD34, CD90, CD44, and CD73 in PBS containing 5% FBS. The fluorescence intensity of these markers was measured using flow cytometry (FACS), and the data were analyzed with FlowJo software. Differentiation potential was assessed using standard protocols. Osteogenesis was evaluated using Alizarin red staining, and adipogenesis was determined using Oil red O staining, confirming the multipotent nature of the isolated cells. These methods were consistent with previously established procedures in our lab (11).
3.2. Isolation and Preparation of Secretomes
To isolate the secretome, WJ-MSCs at the third or fourth passage were cultured in a medium containing 10% FBS until they reached 80% confluence. After washing the cells with PBS, they were maintained in a serum-free culture medium for 20 hours. The conditioned medium was then collected and centrifuged at 4000 g for 10 minutes at 4°C. A second centrifugation of 10 mL of the conditioned medium was performed using Amicon Ultra-15 centrifugal filter units with a 3000 Da molecular weight cut-off membrane (Millipore, USA) at 4°C for two hours. Following the manufacturer’s instructions, the protein concentration in the supernatants was determined using a BCA assay kit from Thermo Scientific (USA). The supernatants were then stored at -80°C until needed (11).
3.3. Cell Culture
The MCF 10A (normal human mammary epithelial cell line) and SKBR-3 (BC cell line with high expression of HER2) cell lines were provided by the National Center for Genetic and Biological Resources of Iran. SKBR-3 cells were grown in DMEM (Gibco, USA) containing 15% fetal calf serum (FCS) and supplemented with an antibiotic mixture (penicillin and streptomycin, Sigma Aldrich). The cells were kept at 37°C in an environment with 95% humidity and 5% CO2. Once they reached 80% confluence, the cells were diluted in serum-free media with varying doses of WJ-MSCs (10, 25, and 50 µg/mL) following a medium change.
3.4. Viability Testing of Cells
To evaluate cell viability, 5 × 10³ cells from each type were seeded into each well of a 96-well plate. After 24 hours, varying concentrations of WJ-MSC secretome (10, 25, and 50 µg/mL) were added to the conditioned medium, and the cells were incubated for another 24 and 48 hours. Cells that were not treated served as the control. At the designated times, each well received 0.5 mg/mL MTT solution (DNA Biotech) and was incubated for 4 hours. The supernatants were subsequently replaced with 100 µL of DMSO, and the absorbance was recorded at 570 nm using an ELISA plate reader (BioRad, CA).
3.5. Colony Assay
MCF10A and SKBR-3 cells were seeded at 500 cells per well in 6-well plates. These cells were then treated with complete media supplemented with 10, 25, and 50 µg/mL WJ-MSC secretome. After 12 days, the colonies were counted by washing the plates, allowing them to air-dry, and staining the cells with a 0.1% crystal violet solution (Lab Grade) for 10 minutes.
3.6. Apoptosis Flow Cytometry Assay
Annexin V-FITC (Pharmingen, San Diego, CA) was used to assess apoptosis in each sample. Variable amounts of WJ-MSC secretomes were applied to each cell culture plate containing 1 × 10⁶ SKBR-3 cells for 48 hours. The cells were then treated with dead cell reagent and Annexin-V. Four types of cells were categorized and counted: Necrotic cells (Annexin-V negative/PI positive), early-stage apoptotic cells (Annexin-V positive/PI negative), late-stage apoptotic cells (Annexin-V positive/PI positive), and healthy cells (Annexin-V negative/PI negative).
3.7. Real-time Polymerase Chain Reaction
After treatment, the Bax/Bcl-2 ratio and the expression levels of Caspase 3, 8, and 9 genes were assessed. RNA was extracted from SKBR-3 cells (1 × 10⁷ cells) using the total RNA Kit (Favorgen, Taiwan). The extracted RNA was then used to synthesize cDNA with the cDNA synthesis kit (Takara, Japan). For each gene, the reaction mixture consisted of 10 µL of SYBER Green reaction mix (Biofact, Korea), 7 µL of nuclease-free water, 1 µL of each forward and reverse primer, and 1 µL of cDNA. Real-time PCR was performed over 40 cycles under the following conditions: The first step was denaturation at 95°C for 10 minutes, followed by denaturation at 95°C for 15 seconds, annealing at 55 - 61°C for 20 seconds, and a final extension at 60°C for 20 seconds. The data were analyzed using the 2-ΔΔCT method, with GAPDH serving as the housekeeping gene (Table 1).
| Target Genes | Primer Sequencing | Annealing Temperature, °C |
|---|---|---|
| GAPDH | ||
| Forward | 5′-GCAAGAGCACAAGAGGAAGA-3′ | 55 |
| Reverse | 5′-ACTGTGAGGAGGGGAGATTC-3′ | 57 |
| Bax | ||
| Forward | 5′-GCTGGACATTGGACTTCCTC-3′ | 57 |
| Reverse | 5′-ACCACTGTGACCTGCTCCA-3′ | 55 |
| Bcl-2 | ||
| Forward | 5′-GGATGCCTTTGTGGAACTGT-3′ | 55 |
| Reverse | 5′-TCACTTGTGGCCCAGATAGG-3′ | 57 |
| Caspase-3 | ||
| Forward | 5′-ATGGTTTGAGCCTGACCAGA-3′ | 55 |
| Reverse | 5′- GGCAGCATCATACACACATAC-3′ | 55 |
| Caspase-8 | ||
| Forward | 5′- ACCTTGTGTCTGAGCTGGTCT-3′ | 57 |
| Reverse | 5′- GCCCACTGATATTCCTCAGGC-3′ | 61 |
| Caspase-9 | ||
| Forward | 5′-GCAGGCTCTGGATCTCGGC- 3′ | 59 |
| Reverse | 5′- GCTGCTTGCCTGTTAGTTCGC-3′ | 57 |
Primers and Their Sequences
3.8. Statistical Analysis
The data were analyzed using one-way ANOVA, followed by post hoc pairwise comparisons with the Bonferroni method. A significance threshold of P < 0.05 was applied. Results are presented as the mean ± standard deviation (SD), with all experiments performed in triplicate.
4. Results
4.1. MTT Assay
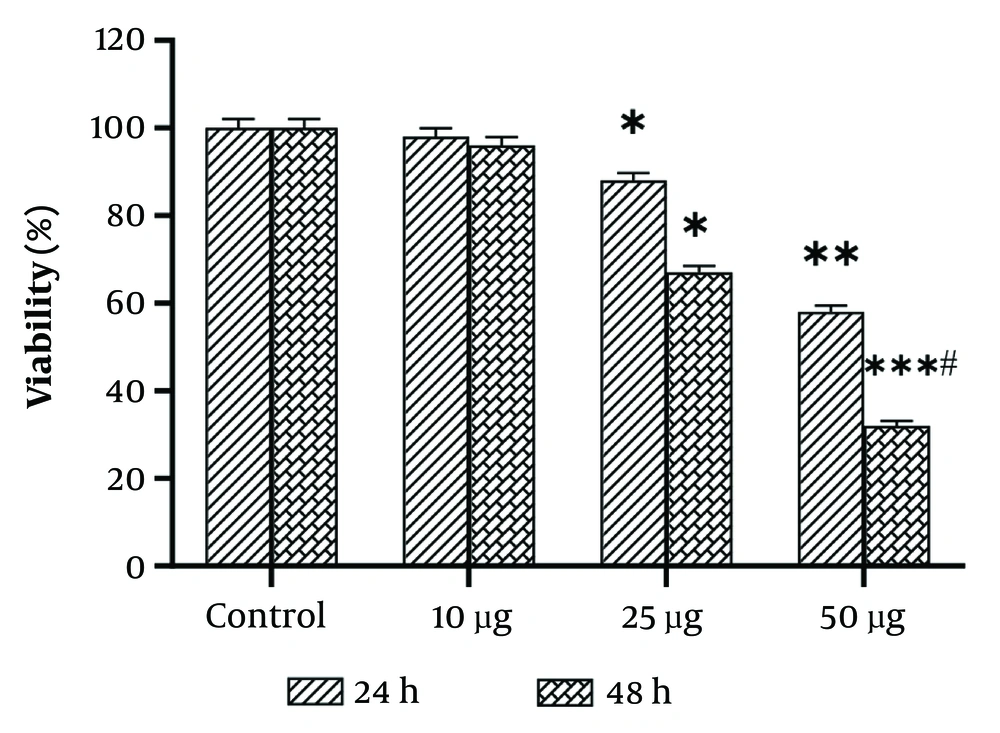
As previously described (9-11), the half-maximal inhibitory concentration (IC50) value of WJ-MSC secretome was determined. After 24 hours of incubation with different dosages of WJ-MSC secretome, as shown in Figure 1, the survival rate significantly decreased. The viability percentage dropped from 100% in the control group to 75.1% and 58% in SKBR-3 cells treated with 25 µg/mL and 50 µg/mL of WJ-MSC secretome, respectively. After 48 hours of incubation with doses of 10 µg/mL and 25 µg/mL of WJ-MSC secretome, no significant differences were detected in the viability percentage of SKBR-3 cells compared to the 24-hour incubation. However, at the lethal dose of 50 µg/mL WJ-MSC secretome, the survival percentage of SKBR-3 cells dropped to 72.2%. The results of the MTT assay indicated that toxic doses of WJ-MSC secretome (25 and 50 µg/mL) were evaluated over 24 hours to assess their cytotoxic effects on SKBR-3 cells. Importantly, WJ-MSC secretome did not significantly affect the viability of MCF10A cells at any of the sub-lethal doses (Table 2).
Viability percentages of SKBR3 cells at various concentrations and incubation times are depicted. Each assay was performed six times (with three replicates for each assay), and the mean ± SD is indicated. Statistical significance is denoted as follows: * P < 0.05, ** P < 0.01, *** P < 0.001. The # symbols indicate comparisons to the control and 25 μg/mL human Wharton's jelly-derived mesenchymal stem cells (hWJMSCs)-Se groups, respectively.
| Groups | Control | 10 µg | 25 µg | 50 µg |
|---|---|---|---|---|
| Viability (%) | 100 ± 0.00 | 100 ± 0.00 | 98.2 ± 1.1 | 98.8 ± 1.3 |
| Counts of colonies | 1.9 ± 0.11 | 1.1 ± 0.11 | 1.15 ± 0.11 | 1.55 ± 0.19 |
| Early-stage apoptosis (%) | 1.01 ± 0.17 | 1.01± 0.18 | 1.04 ± 0.21 | 1.11 ± 0.25 |
| Late-stage apoptosis (%) | 1.9 ± 0.11 | 1.7 ± 0.14 | 1.29 ± 0.24 | 1.25 ± 0.19 |
The Outcomes Derived from the MTT Analysis, Colony Forming Unit Assay, and Annexin V/PI Technique for MCF 10A Treated Cells (n = 5) a
4.2. Colony Assay
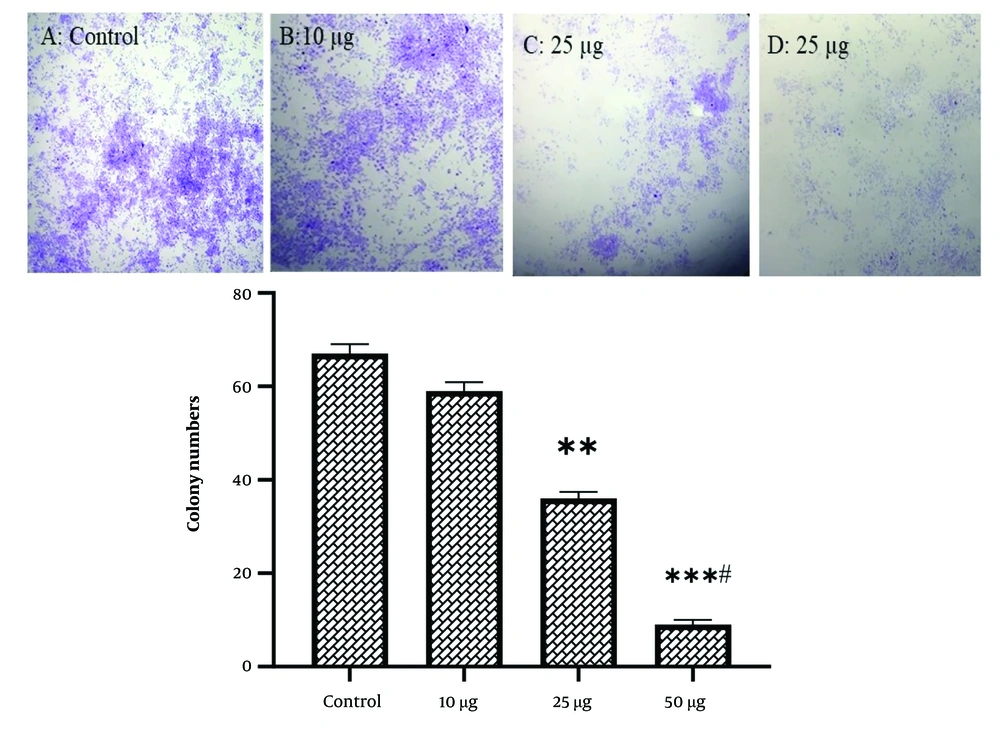
In SKBR-3 cells, treatment with 25 µg/mL WJ-MSC secretome significantly decreased colony formation (P < 0.01). Figure 2 shows that the use of 50 µg/mL WJ-MSC secretome as a conditioned medium led to a substantial decrease in the number of colonies compared to control cells (P < 0.001) and cells treated with 25 µg/mL (P < 0.01). WJ-MSC secretome did not significantly affect colony formation in MCF10A cells at any dose (Table 2).
Colony formation and the number of SKBR3 cells in various groups are presented. Each assay was conducted six times (with three replicates for each assay), and the mean ± SD is provided. Statistical significance is denoted as follows: ** P < 0.01, *** P < 0.001. The # symbol indicates comparison to the control and concentrations, while the ## symbol indicates comparison to the 25 μg/mL and 50 μg/mL human Wharton's jelly-derived mesenchymal stem cells (hWJMSCs)-Se groups, respectively.
4.3. Assessment of Apoptosis in SKBR-3 Cells Following Wharton's Jelly Mesenchymal Stem Cells Secretome Treatment
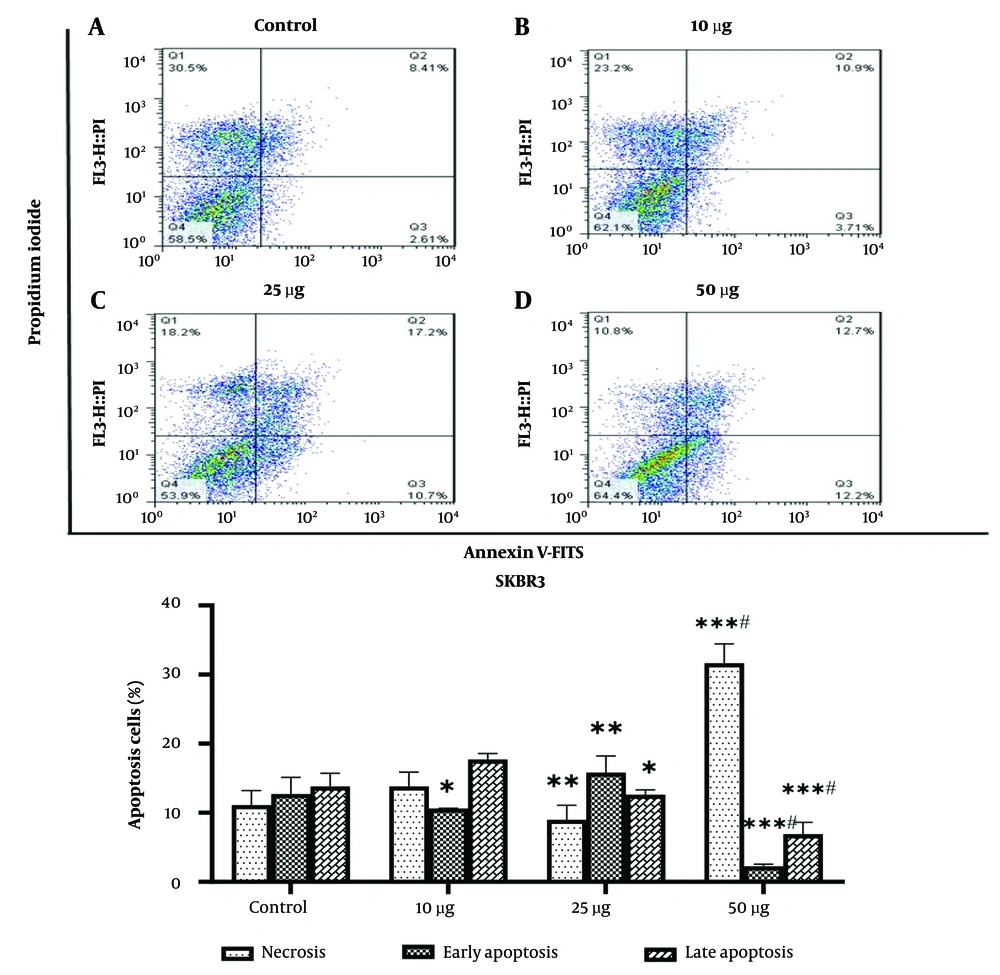
Induction of apoptosis in SKBR-3 cells by WJ-MSC secretome was assessed using FACS analysis with annexin V/PI staining. The fraction of apoptotic cells increased in a dose-dependent manner following WJ-MSC secretome treatment. In untreated cells, 31.82% were necrotic, 6% were in late apoptosis, 2% were in early apoptosis, and 59.2% remained viable. The percentage of viable cells increased from 59.2% to 66.2% and 62.2% at concentrations of 25 µg/mL and 50 µg/mL WJ-MSC secretome, respectively, compared to the control group. Exposure to 25 µg/mL WJ-MSC secretome resulted in a significant increase in both early (a 3.3-fold increase, P < 0.05) and late (a 1.6-fold increase, P < 0.05) apoptosis relative to the control. A concentration of 50 µg/mL WJ-MSC secretome markedly enhanced early (a 6.3-fold increase, P < 0.01) and late (a 2.1-fold increase, P < 0.01) apoptosis in SKBR-3 cells compared to the control group (Figure 3). Notably, WJ-MSC secretome had minimal to no effect on the apoptotic indices of MCF10A cells at both doses (Table 2).
A, hWJMSCs-Se induced apoptosis in SKBR3 cells; B, each assay was conducted in triplicate. Representative dot plots of annexin V-FITC fluorescence (X-axis) versus PI fluorescence (Y-axis) for both control and experimental groups are shown. The lower left quadrant represents live cells, the lower right quadrant indicates early apoptosis, the upper right quadrant denotes late apoptosis, and the upper left quadrant corresponds to necrotic cells. Apoptotic and necrotic indices are also displayed. Each assay was performed three times, with the mean ± SD provided. Statistical significance is represented as follows: * P < 0.05, ** P < 0.01, *** P < 0.001, # P < 0.05. The asterisk (*) indicates comparisons to the control and concentrations, while the hash (#) symbol denotes comparisons between the 25 μg/mL and 50 μg/mL human Wharton's jelly-derived mesenchymal stem cells (hWJMSCs)-Se groups.
4.4. Quantitative Real-time Polymerase Chain Reaction
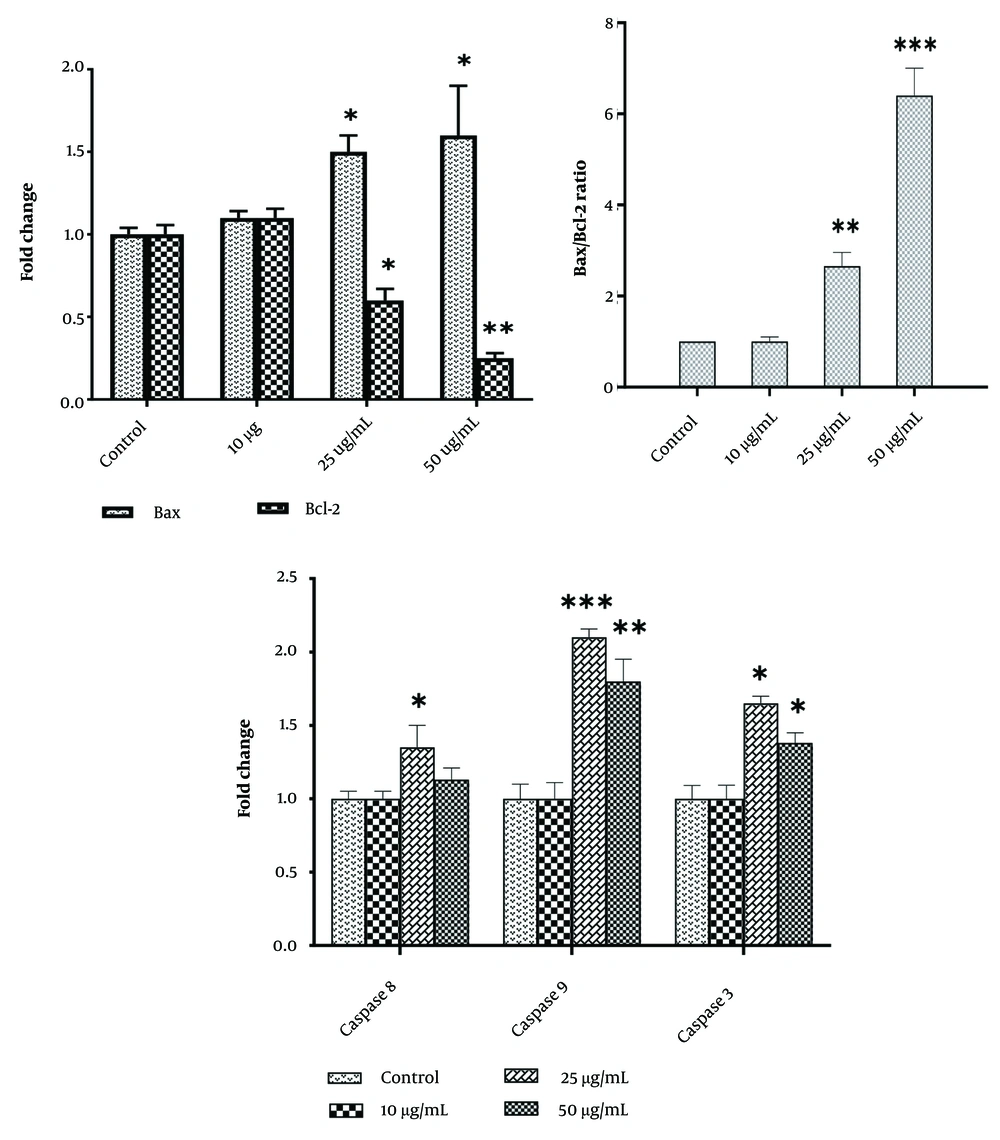
At a dose of 25 µg/mL, real-time PCR showed that WJ-MSC secretome significantly decreased the Bcl-2 gene level while considerably increasing the Bax gene expression level. The levels of Bax and Caspase-3, -8, and -9 expression were significantly increased by a 50 µg/mL dose of WJ-MSC secretome compared to the control and 25 µg/mL WJ-MSC secretome-treated groups (Figure 4).
Effects of hWJMSCs-Se on gene expression. Expression levels are presented as fold change relative to control untreated cells. Each assay was conducted five times, with the mean ± SD provided. Statistical significance is indicated as follows: * P < 0.05, ** P < 0.01, and *** P < 0.001, representing comparisons to the control and 25 μg/mL human Wharton's jelly-derived mesenchymal stem cells (hWJMSCs)-Se groups, respectively.
5. Discussion
The current investigation demonstrated that the viability percentage of the HER2-positive BC cell line was significantly reduced in the 25 μg/mL group after 48 hours and in the 50 μg/mL group at both 24 and 48 hours. Additionally, colony formation and the number of SKBR3 cells were markedly diminished in the 50 μg/mL group. WJ-MSC secretome induced early apoptosis more effectively in the 25 μg/mL group than in the 50 μg/mL group. This was demonstrated by the enhanced expression of apoptotic genes, including Caspase-3 and Caspase-9, along with a parallel reduction in the anti-apoptotic gene Bcl-2. Moreover, a lower Bax/Bcl-2 ratio was observed in the 25 μg/mL group compared to the 50 μg/mL group.
The results showed that WJ-MSC secretome in both the 25 μg/mL and 50 μg/mL groups reduced the viability and proliferation of the SKBR3 cell line in a concentration- and time-dependent manner. The time- and dose-dependent inhibitory activity of WJ-MSC secretome on viability was evident across multiple cancer cell lines, including those from breast cancer such as MDA-MB-231, T47D, and MCF7 (12, 13); ovarian carcinoma TOV-112D (14); osteosarcoma MG-63 (15); benign neoplastic keloid cells (16); bladder cancer; lymphoma cells; gastric cancer cells; leukemia cells; human endometrial stromal cells; and colon cancer cells HT-29 (11). Animal studies further supported the inhibitory effects of WJ-MSC secretome on the development of osteosarcoma, lung cancer, BC, and pancreatic cancer (17). Contrasting results were observed in glioblastoma cell lines U251 and SNB-19, where WJ-MSC secretome promoted high cell survival, proliferation, and migration (18). However, other studies have shown that WJ-MSC secretome inhibits the growth and proliferation of the U87MG glioma cell line, emphasizing the impact of cell type and culture techniques on the outcomes (19).
The WJ-MSC secretome at 25 μg/mL showed a higher early-to-late apoptosis ratio compared to 50 μg/mL (Figure 3). Early apoptosis (annexin V+/PI−) reflects the initial stages of apoptosis with intact membrane integrity, while late apoptosis (annexin V+/PI+) indicates membrane loss and cell death progression. This suggests that 25 μg/mL induces apoptosis more gradually, allowing cells time for apoptotic signaling before reaching terminal stages (20). At 50 μg/mL, the lower early-to-late apoptosis ratio suggests an accelerated apoptotic process due to a stronger apoptotic signal. This aligns with previous studies demonstrating the anti-tumor effects of WJ-MSC secretome on ovarian, lung, and leukemic cancer cells (14, 21).
Human Wharton's jelly-derived mesenchymal stem cells offer benefits such as low immunogenicity, multipotency, and non-carcinogenicity. Their anticancer effects involve both direct contact and secreted factors, with conditioned media shown to inhibit tumor growth. Modified MSCs produce immunostimulants (IL2, IL7, IL12, CX3CL1) and apoptosis-inducing molecules such as TRAIL, IL-8, and NK4. Cytokines in the WJ-MSC secretome, such as TRAIL, suppress cell growth via the Bax and Caspase-3 pathways, while TNFα induces necrotic or apoptotic cell death depending on the context (22, 23).
In our study, the WJ-MSC secretome at 25 μg/mL, compared to the 50 μg/mL group, significantly influenced the expression of Bax and its subsequent downregulation of the anti-apoptotic gene Bcl-2, thereby promoting the intrinsic apoptotic pathway. The Bax/Bcl-2 ratio is crucial in determining cell survival or apoptosis, with a lower Bax/Bcl-2 ratio promoting apoptosis and a higher ratio contributing to cell survival and cancer progression (24). Notably, the secretome induces apoptosis in colon cancer cells through the activation of the intrinsic pathway (11). Figure 4 underscores the impact of WJ-MSC secretome on the levels of Caspase-9 and Caspase-3 gene expression in the SKBR3 cell line. These findings are consistent with previous research demonstrating the ability of WJ-MSC secretome to induce apoptosis in MDA-MB 231 and MCF7 cell lines (25). Furthermore, WJ-MSC secretome treatment significantly increased the mRNA expression of Caspases-9 and -3 in a dose-dependent manner, supporting its apoptotic effects.
The observed anticancer effect of WJ-MSC secretome is attributed to an apoptotic mechanism. These studies suggest that WJ-MSCs induce apoptosis through multiple mechanisms and signaling pathways. This includes the reduction of apoptosis in nucleus pulposus cells in intervertebral disc degeneration by inhibiting Wnt/β-catenin signaling (26), inhibition of gastric cancer cell growth and viability by inducing apoptosis and modifying NF-κB and MAPK signaling pathways (27), and protection against sepsis-induced organ injury by reducing systemic inflammation, partially through activation of the cholinergic anti-inflammatory pathway (28). Additionally, previous studies have demonstrated that MSCs derived from the human umbilical cord can trigger apoptosis in glioma and xenografted cells by activating Caspase-3 and Caspase-9 (29). These findings are further supported by the enhancement of apoptotic gene expression, including Caspase-3 and Caspase-9, and the subsequent downregulation of the anti-apoptotic gene Bcl-2. The differential Bax/Bcl-2 ratio observed between the two concentrations reinforces the notion that the apoptotic response is dose-dependent, with higher concentrations promoting a faster apoptotic pathway (30).
The implications of these findings are critical for therapeutic strategies, as they suggest that varying concentrations of WJ-MSC secretome can modulate the kinetics of apoptosis in cancer cells. A controlled apoptotic response, as seen in the 25 μg/mL group, may be beneficial in a clinical setting where gradual induction of cell death is preferable to minimize adverse effects on surrounding tissues. On the other hand, a higher concentration leading to rapid apoptosis might be advantageous for swiftly reducing tumor burden in aggressive cancers.
Overall, the ability to fine-tune the apoptotic response through precise dosing of WJ-MSC secretome offers a promising avenue for targeted breast cancer therapy. Future studies should focus on understanding the molecular mechanisms governing this differential apoptotic induction and evaluating the in vivo efficacy and safety of WJ-MSC secretome at various concentrations.
5.1. Conclusions
This study examined the effects of WJ-MSC secretome on HER2-positive breast cancer cells. Our findings indicated that WJ-MSC secretome significantly reduced the viability and growth of SKBR3 cells in relation to both dosage and duration. Additionally, WJ-MSC secretome promoted early apoptosis in a dose- and time-dependent manner, as evidenced by alterations in the expression of apoptotic genes, including Caspase-3 and Caspase-9, as well as changes in the ratio of pro-apoptotic Bax to anti-apoptotic Bcl-2. These results suggest that the timing and dosage of WJ-MSC secretome administration can substantially influence the apoptotic process in cancer cells. Furthermore, our findings highlight the potential of WJ-MSC secretome as a novel and effective therapeutic strategy for targeted breast cancer treatment. Future studies should aim to further elucidate the molecular mechanisms underlying this phenomenon and explore the efficacy and safety of WJ-MSC secretome at various doses and time points.