1. Background
Obesity is a major cause of metabolic diseases (1). According to the World Health Organization’s report, by 2035, more than half of the world's population is expected to be overweight or obese. Common causes of obesity include lifestyle changes, physical inactivity, and the consumption of high-fat diets (2). The development of obesity and weight gain predisposes individuals to diseases such as hypertension, elevated blood lipids, cardiovascular conditions, metabolic bone disorders, and insulin resistance (3).
Insulin resistance, a consequence of obesity and overweight, occurs due to a reduced response of cellular receptors to insulin in the bloodstream. This condition leads to increased blood sugar and free fatty acid levels. In response, pancreatic beta cells must secrete more insulin to facilitate glucose entry into cells, resulting in abnormally high blood insulin levels (4).
Additionally, research findings suggest that bones can function as an endocrine organ involved in regulating glucose and energy metabolism. Bone-related proteins, particularly osteocalcin (OC), have been shown to play a role in insulin resistance (5).
Osteocalcin is a non-collagenous organic protein involved in bone metabolism, secreted by osteoblasts and subject to carboxylation after translation. In its uncarboxylated form, it stimulates beta cells and the expression of adiponectin in fat cells, improving insulin secretion and sensitivity (6). Uncarboxylated osteocalcin (ucOC), composed of 49 amino acids in humans, functions as a hormone regulating glucose and energy metabolism in pancreatic beta cells, adipose tissue, and muscle tissue (7). Furthermore, ucOC has been reported to affect white fat tissue by inducing the expression of genes associated with energy expenditure, thereby increasing insulin sensitivity (8).
In this context, Razny et al. (9) concluded in a study comparing prediabetic and healthy individuals that ucOC is negatively correlated with fasting insulin and glucose levels, and its concentration is lower in prediabetic individuals. Similarly, Mohammad Rahimi et al. (10) examined the effects of aerobic interval training, resistance training, and concurrent training conducted three times a week for 12 weeks on serum leptin levels, ucOC, and adiponectin in obese men. The study found that leptin levels increased more after aerobic interval and concurrent training, while ucOC significantly increased only in the concurrent and aerobic interval training groups. Adiponectin levels, however, showed significant increases across all three exercise modalities. Additionally, concurrent training resulted in a more pronounced reduction in glucose levels, insulin levels, and the Insulin Resistance Index compared to the other two exercise groups.
Regular physical activity is a non-pharmacological method to enhance insulin sensitivity, increase ucOC levels, and combat obesity. While most exercise recommendations for weight loss emphasize continuous aerobic exercise, this form of exercise is often uniform (11). As a result, alternative methods, such as interval training, have been proposed as potentially easier for obese individuals to adhere to (12). Moreover, interval training has been shown to lead to desirable changes, including improvements in maximal oxygen consumption, blood glucose levels, insulin levels, Insulin Resistance Index, HbA1c, body mass, and BMI in obese individuals (12, 13).
Levinger et al. (14) explored the effects of 30 minutes of high-intensity interval exercise on ucOC and insulin sensitivity in 11 obese men. The results demonstrated that high-intensity exercise increased ucOC and improved insulin sensitivity. However, there is still no consensus regarding the optimal intensity, type, or timing of exercise. While most recommendations for improving insulin sensitivity focus on aerobic and resistance exercises (12), interval training appears to offer superior benefits for weight reduction, improved insulin sensitivity, and increased ucOC levels in obese individuals (10, 13, 15, 16).
In summary, the effects of different exercise activities on ucOC levels and insulin resistance have produced limited and conflicting results (14, 17, 18). For instance, while a study by Levinger et al. examining a single exercise session (14) and another by Mohammad Rahimi et al. on a 12-week aerobic exercise program (10) reported significant increases in ucOC levels, no significant changes were observed after eight weeks of resistance exercises (17).
Given that obesity and insulin resistance are critical public health concerns, and exercise interventions are increasingly recognized as effective non-pharmacological strategies for metabolic improvement, it is crucial to further explore the impact of exercise on these conditions. The metabolic improvements observed in overweight and obese individuals following exercise may be linked to the effects of exercise on ucOC levels and insulin resistance markers. However, previous studies have largely focused on general insulin resistance markers without specifically addressing the role of OC in metabolic health, particularly in obese women.
While high-intensity interval training (HIIT) has been extensively studied, this research emphasizes moderate-intensity interval training. This approach may be more suitable for individuals with lower fitness levels or health concerns, thereby increasing the practical applicability of the findings to a broader population. Given the scarcity of research in this area, this study seeks to determine whether eight weeks of moderate-intensity interval training can induce changes in ucOC levels and insulin resistance in obese women.
2. Objectives
This study aims to investigate the effect of eight weeks of moderate-intensity interval training on uncarboxylated OC levels and insulin resistance markers in obese women.
3. Methods
3.1. Subjects
This practical and semi-experimental study was conducted using an experimental group and a control group with pre-test and post-test designs. The statistical population comprised middle-aged obese women in Mashhad. In 2023, a public call was announced in Mashhad inviting volunteer women to participate in the study. The research sample included 20 middle-aged women aged 35 - 45 years with a Body Mass Index (BMI) of 35 - 45 kg/m². Participants were randomly assigned to either the control group (n = 10) or the moderate-intensity interval training group (n = 10).
Participants in the control group were instructed to maintain their usual levels of physical activity and avoid engaging in any new exercise programs throughout the study. Additionally, all participants were asked to follow their regular diets.
At the beginning of the study, the nature of the collaboration, benefits, and potential risks of participation were clearly explained to the volunteers. They were informed that they could withdraw from the study at any time without any obligation to continue. All collected information was kept confidential, with the researchers publishing only general and group-level results without mentioning names or personal details.
The inclusion criteria required participants to have a BMI of 30 - 35 kg/m², no history of regular physical activity in the six months prior to the study, no chronic diseases (e.g., cardiovascular, renal, or thyroid disorders), and to be non-smokers. The exclusion criteria included missing two or more exercise sessions, the onset of cardiovascular, renal, or hepatic diseases during the eight-week program, neuromuscular disabilities that hindered exercise performance, or a history of smoking.
Participants voluntarily joined the study after meeting the eligibility criteria and signed an informed consent form. All research activities adhered to the principles of the Helsinki Declaration, and ethical considerations were reviewed and approved by the research ethics committee at Hakim Sabzevari University with the code IR.HSU.REC.1402.017.
All exercise sessions were conducted under the supervision of an exercise physiologist to ensure proper technique and safety.
3.2. Body Composition
At the beginning and after the eight-week intervention, several anthropometric indices of the participants were assessed. Body weight (kg) and height (cm) were measured using a Digital Scale and a stadiometer (Seca, Germany), respectively. Body Mass Index was calculated by dividing body weight (kg) by the square of height (m²). Waist circumference was measured at the narrowest point of the waist, and hip circumference was measured at the widest point of the hips using a non-stretchable tape measure without applying pressure. The waist-to-hip ratio was determined by dividing the waist circumference by the hip circumference.
3.3. Blood Sampling
Blood samples were collected from the participants in two stages: The first before the commencement of the exercise program and the second 48 hours after the final exercise session. Both blood draws were performed by a laboratory specialist. Five milliliters of blood were taken after 8 to 10 hours of fasting under consistent conditions between 8:00 and 10:00 AM from the antecubital vein. The samples were collected in tubes containing K2EDTA and allowed to rest for 15 minutes before analysis.
The serum was separated by centrifugation at 3000 RPM for 10 minutes and stored at -80°C until analysis. After sample collection, post-tests were conducted to measure ucOC levels using a kit from Padgin Teb Company, purchased from ZellBio, Germany. The kit had a coefficient of variation of less than 10% and a sensitivity of less than 0.5 pg/mL (serial number ZB-15126C-H9648).
Blood glucose levels were estimated using the Pars Azmoon kit (Iran) via an enzymatic method, which had a sensitivity of less than 2 mg/dL and a coefficient of variation of less than 1.82%. Insulin levels were determined using the ELISA method and the Saman Tejharat Noor kit (CAT: K2B158, Tehran, Iran). The Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated using the following formula (19):
HOMA-IR = Fasting insulin (µL/mL) × Fasting glucose (mg/dL)/405
3.4. Physical Fitness Test
Participants were asked to measure their maximum oxygen consumption (VO₂max). After a warm-up session, they were instructed to walk 1609 meters (one mile) at the fastest possible speed. Their heart rates were recorded before and immediately after completing the walk. The following formula, which incorporates body weight in pounds (1 pound = 453 g), age in years, gender factor (men = 1, women = 0), time to complete one mile in minutes, and the immediate post-test heart rate in beats per minute, was used to calculate VO₂max:
VO₂max equation (mL.kg-1.min-1) = (132.853 - 0.0769 × weight) – (0.3877 × age) + (6.318 × gender) – (3.2699 × time) – (0.1565 × heart rate)
3.5. Interval Training Program
The moderate-intensity interval training program consisted of a structured routine with 10 minutes of warm-up at the beginning and 10 minutes of cool-down at the end of each session. Warm-up activities included stretching, rhythmic movements, and light aerobic exercises.
The training lasted eight weeks, with three sessions per week. Each session was 20 to 40 minutes long, conducted at an intensity equivalent to 50 - 75% of the participants' maximum heart rate. Maximum heart rate was calculated using the formula:
Maximum heart rate = 220 - age
Exercise intensity was monitored with a Polar heart rate monitor (20). At the end of each session, participants performed a 10-minute cool-down involving slow running, walking, and stretching to help the body return to its resting state.
At the conclusion of the eight-week program, all measurements were repeated under the same conditions as the pre-test, and the data was collected for analysis.
3.6. Statistical Analysis
The collected data were entered into SPSS software version 26 for analysis. The normality of the data was assessed using the Shapiro-Wilk test, while the equality of variances between the groups was evaluated with Levene's test. To analyze intra- and inter-group changes, a one-way analysis of variance (ANOVA) with repeated measurements was employed. A significance level of P < 0.05 was set as the threshold for determining statistically significant differences.
4. Results
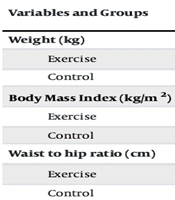
The characteristics of the participants based on pre-test results showed that the average age in the exercise group was 39.00 ± 4.02 years, height 162.10 ± 6.41 cm, weight 82.83 ± 7.21 kg, and BMI 31.50 ± 1.82 kg/m². In the control group, the average age was 41.40 ± 5.75 years, height 158.00 ± 5.09 cm, weight 81.24 ± 12.44 kg, and BMI 32.47 ± 4.21 kg/m². The results in Table 1 indicate that intra-group changes in average body weight (P = 0.016), BMI (P = 0.015), and waist-to-hip ratio (P = 0.001) showed significant decreases by the end of the exercise period. Additionally, the time interaction changes between groups for the waist-to-hip ratio variable were statistically significant (P = 0.001).
| Variables and Groups | Stages | ||||||
|---|---|---|---|---|---|---|---|
| Pre-Test | 8th Week | Percentage of Changes | P-Value b | Time P-Value | Group P-Value | Time × Group P-Value | |
| Weight (kg) | 0.021 | 0.731 | 0.949 | ||||
| Exercise | 82.83 ± 7.21 | 81.32 ± 7.40 | -1.85 | 0.016 c | |||
| Control | 81.24 ± 12.44 | 79.65 ± 13.41 | -1.99 | 0.187 | |||
| Body Mass Index (kg/m2) | 0.002 | 0.546 | 0.918 | ||||
| Exercise | 31.50 ± 1.82 | 30.94 ± 2.06 | -1.80 | 0.015 c | |||
| Control | 32.47 ± 4.21 | 31.86 ± 4.79 | -1.91 | 0.178 | |||
| Waist to hip ratio (cm) | 0.135 | 0.099 | 0.001 c | ||||
| Exercise | 0.85 ± 0.05 | 0.79 ± 0.03 | -7.59 | 0.001 c | |||
| Control | 0.86 ± 0.06 | 0.87 ± 0.05 | 1.14 | 0.199 | |||
Comparison of Intra- and Inter-Group Mean Changes in Body Composition in Sedentary Women a
The results in Table 2 reveal that intragroup changes in average ucOC levels (P = 0.005) showed a significant increase by the end of the exercise period. Furthermore, insulin levels and the Insulin Resistance Index significantly decreased, while glucose levels experienced a non-statistically significant decrease of 3.96%. Maximal oxygen consumption showed a significant increase (P = 0.001) at the end of the exercise period.
| Variables and Groups | Stages | ||||||
|---|---|---|---|---|---|---|---|
| Pre-Test | 8th Week | Percentage of Changes | P-Value b | Time P-Value | Group P-Value | Time × Group P-Value | |
| Uncarboxylated osteocalcin (μmol/L) | 0.001 | 0.832 | 0.193 | ||||
| Exercise | 145.63 ± 37.23 | 166.38 ± 33.03 | 12.47 | 0.005 c | |||
| Control | 153.45 ± 16.06 | 163.37 ± 11.00 | 6.07 | 0.089 | |||
| Glucose (mg/dL) | 0.573 | 0.03 | 0.012 c | ||||
| Exercise | 86.50 ± 9.84 | 83.20 ± 9.76 | -3.96 | 0.098 | |||
| Control | 94.60 ± 13.83 | 100.22 ± 14.46 | 5.60 | 0.072 | |||
| Insulin (microunit/L) | 0.205 | 0.278 | 0.007 c | ||||
| Exercise | 14.85 ± 8.26 | 7.41 ± 4.17 | -100.40 | 0.009 c | |||
| Control | 12.41 ± 9.21 | 16.08 ± 8.21 | 22.82 | 0.287 | |||
| HOMA-IR | 0.322 | 0.165 | 0.009 c | ||||
| Exercise | 3.23 ± 1.91 | 1.53 ± 0.88 | -111.11 | 0.008 c | |||
| Control | 3.11 ± 2.93 | 4.14 ± 2.61 | 24.87 | 0.278 | |||
| Max oxygen consumption (ml/kg/min) | 0.001 | 0.206 | 0.014 c | ||||
| Exercise | 24.42 ± 2.18 | 26.41 ± 1.71 | 7.53 | 0.001 c | |||
| Control | 26.79 ± 3.26 | 27.17 ± 3.44 | 1.39 | 0.398 | |||
Comparison of Intra- and Inter-Group Mean Changes in Uncarboxylated Osteocalcin Levels and Sugar Markers in Sedentary Women a
Regarding changes between groups, the time interaction effects for fasting glucose (P = 0.012), fasting insulin (P = 0.007), and the Insulin Resistance Index (P = 0.009) were statistically significant. However, no significant change was observed between groups in ucOC levels (P = 0.193). Time interaction effects between groups for maximal oxygen consumption were statistically significant.
5. Discussion
Based on the results obtained from the changes between different groups, the time interaction changes in the group for the ucOC variable were not statistically significant. However, the results of the comparison of means within the groups indicate a significant increase in ucOC. These findings are consistent with those of Mohammad Rahimi et al. (10), but not with those of Colleluori et al. (21). Mohammad Rahimi et al. (10) investigated the effect of 12 weeks of exercise on serum adiponectin, ucOC, and adiponectin in 44 obese men with metabolic syndrome, who were randomly assigned to aerobic interval exercise, resistance exercise, concurrent exercise, or a control group. The research results showed that after aerobic interval and concurrent exercise, OC increased significantly. However, adiponectin increased significantly in all three exercise groups. On the other hand, concurrent exercise resulted in a greater reduction in glucose, insulin, and Insulin Resistance Index compared to the other two groups. In contrast, Colleluori et al. (21) examined the effect of weight loss, exercise, or both on the secretion of ucOC and insulin in obese elderly individuals. The diet group followed a low-calorie, high-protein diet, while the exercise group performed flexible, aerobic, resistance, and balance exercises three times a week, and the diet-exercise group engaged in both interventions. The results showed no significant changes in ucOC levels or the Insulin Resistance Index in any group. One possible reason for the discrepancy in results between the present study and that of Colleluori et al. (21) could be attributed to differences in the exercise period, intensity, and participants' characteristics. Moreover, in the study by Colleluori et al. (21), the training content consisted of a combination of aerobic, resistance, balance, and flexibility exercises, while the present study focused on moderate-intensity interval training.
There is a strong association between ucOC and physical activity. Physical activity, particularly exercises that involve body weight-bearing, stimulates OC production by increasing osteoblast activity. Weight-bearing exercises, such as running or resistance training, apply mechanical pressure to the bones, which serves as a key stimulus for osteoblast activity and bone formation.
During exercise, the body experiences physiological stresses that create a slightly acidic environment. This acidic environment inhibits OC carboxylation, leading to an increase in ucOC levels in the bloodstream. Uncarboxylated osteocalcin appears to have multiple beneficial effects on overall metabolic health, fitness, exercise performance, and recovery. The increase in ucOC levels associated with exercise may offer several health benefits. As a metabolic hormone, ucOC improves insulin sensitivity and glucose metabolism, which can be useful for diabetes prevention and management (22). It also provides the energy needed for muscle contraction and function.
Undercarboxylated osteocalcin stimulates the secretion of adiponectin, a hormone that improves fat metabolism, which is crucial during prolonged exercise. As a result, it increases energy expenditure, aiding in weight management and obesity prevention (23). The relationship between muscle and ucOC in energy metabolism and exercise adaptation is significant. Undercarboxylated osteocalcin, or bioactive OC, is released by osteoblasts and has been shown to directly impact muscle energy metabolism during exercise. When ucOC is released during exercise, it binds to the GPRC6A receptor in myofibrils, promoting the absorption and utilization of nutrients (24).
This includes several key effects on muscle function: It enhances the expression of fatty acid transporters and stimulates beta-oxidation, leading to increased use of fatty acids. It also strengthens the translocation of the glucose transporter GLUT4 to the plasma membrane, increasing glucose uptake and metabolism in muscle cells. This direct effect of ucOC on muscle energy metabolism is crucial for nutrient absorption and utilization during exercise, ultimately contributing to overall exercise adaptation.
In addition, there is evidence of a feedback loop between bone (via OC) and muscle (via IL-6) that enhances exercise adaptation through co-stimulatory mechanisms. This further emphasizes the complex relationship between bone-derived hormones and muscle function during physical activity (25, 26). Osteocalcin signaling in myofibers upregulates the secretion of IL-6, a known target gene of OC in muscle, which in turn stimulates lipolysis and fat oxidation, significantly increasing during physical activity. This leads to improved exercise capacity. Furthermore, OC production by osteoblasts and its activation by bone resorption are increased (25).
This myokine supports the production of decarboxylated osteocalcin through signaling in bone cells, leading to increased expression of RankL (a cytokine important for osteoclast differentiation) and decreased osteoprotegerin (Opg), which inhibits bone resorption in cultured osteoblasts. This suggests that IL-6 may act on osteoblast lineage cells to increase bone resorption during exercise (27).
Based on the results obtained from the changes between different groups, the time interaction changes in fasting glucose, fasting insulin, and insulin resistance are statistically significant. The results of the comparison of means within the groups show a significant decrease in fasting insulin levels and the Insulin Resistance Index. These findings are consistent with the results of Huifen et al. (28) and Zeng et al. (29), but not with those of Sari-Sarraf et al. (30).
Huifen et al. (28) concluded, after examining the effects of a moderate-intensity resistance training program on blood glucose levels and other health-related indicators in patients with gestational diabetes, that blood glucose and insulin levels were lower after the intervention compared to before the intervention. Similarly, Zeng et al. (29) found that eight weeks of moderate-intensity endurance training combined with a medium-carbohydrate, low-fat, calorie-restricted diet significantly reduced HbA1c levels, 2-hour post-intervention glucose levels, fasting insulin, HOMA-IR, HOMA-IS, and body fat percentage.
In contrast, Sari-Sarraf et al. (30) reported that four weeks of combined aerobic and resistance exercises, along with flaxseed supplementation, did not result in significant changes in insulin, insulin resistance, or blood glucose levels in overweight girls. One reason for the differing results could be the duration, intensity, and content of the exercise programs. Insulin sensitivity is closely related to physical activity, and it has been shown that physical exercise improves insulin sensitivity in insulin-resistant individuals. Exercise increases PI3-k activation through IRS-1, enhancing the efficiency of the insulin pathway without affecting the expression of insulin cascade components. This suggests that physical exercise improves insulin sensitivity by intermittently increasing insulin receptor signaling or by enhancing insulin receptor sensitivity (31).
It is noteworthy that physical exercises do not increase the ability to stimulate insulin for enhancing PI3-k activity through IRS-1, but they do increase GLUT-4 protein expression by up to 22%, which is associated with increased Akt protein expression. It is important to note that Akt phosphorylation inhibits GSK-3β activity, leading to the stimulation of gene transcription and protein synthesis. Therefore, it is reasonable to hypothesize that improved insulin sensitivity in response to chronic exercise is primarily driven by the regulation of transcription processes, and physical exercise is also accompanied by an anti-inflammatory response. However, there are still conflicting results regarding the type and intensity of exercise required to achieve an anti-inflammatory effect (31).
Studies have shown that exercise interventions, including aerobic, resistance, and combined aerobic exercises with calorie restriction for weight loss, have the most beneficial effects on insulin sensitivity and glucose homeostasis markers. Moderate-intensity aerobic exercise has been shown to provide more benefits for skeletal muscle insulin sensitivity, as measured by intravenous glucose tolerance tests, pancreatic β-cell function in the early phase, and glucose tolerance, as assessed through oral glucose tolerance tests. In comparison to moderate-intensity exercises, higher-intensity exercises seem to have a stronger impact on peripheral insulin sensitivity, evaluated during hyperinsulinemia-euglycemic clamps (32).
Improvements in insulin sensitivity due to exercise appear to involve the activation of transcription factors, which regulate the expression and suppression of target genes, ultimately altering metabolic properties. Further research at the cellular level is necessary to better understand the molecular basis of improved insulin signaling resulting from exercise (31).
Contradictory results are likely due to differences in participants' physiological conditions, health, exercise duration, type and intensity, and nutritional status. Given the numerous limitations of this study, including diverse diets, varied adaptation responses to physical activity, a small number of participants due to dropouts, and individual differences, caution must be exercised when interpreting the results.
5.1. Conclusions
In conclusion, it can generally be stated that moderate-intensity interval exercises significantly increase ucOC concentration and decrease fasting insulin and Insulin Resistance Index in obese women. Additionally, a significant increase in maximal oxygen consumption levels was observed in the participants at the end of the exercise intervention period. Considering the significant increase in ucOC concentration, it is possible that, by affecting beta cells, it could lead to improvements in factors influencing insulin resistance. However, given the importance of physical activity in preventing and treating obesity-related diseases, experts recommend exercise counseling to reduce the incidence of associated illnesses. Due to the numerous limitations of this study, including diverse diets, varied adaptation responses to physical activity, a small number of participants due to dropouts, and individual differences, caution should be exercised when interpreting the results.