1. Background
Genetics, environmental factors, lifestyle, and nutrition all play a role in cancer development. The initial stage involves proliferation and growth without proper cell control due to an inadequate response to natural growth inhibitors and cell cycle regulation (1). In the United States, cancer is the second leading cause of death. From 2005 to the present, cancer rates have risen from 7.6 million to 11 million cases per year, and it is projected to exceed 15.5 million by 2030 (2). Breast cancer is the most commonly diagnosed cancer among women worldwide (3). Cancer treatment methods include surgery, radiation therapy, chemotherapy, gene therapy, hormone therapy, and others. Among these, chemotherapy is one of the most effective methods (4). This treatment uses drugs that inhibit or slow down the division of cancer cells. However, healthy cells are also damaged by chemotherapy drugs. Consequently, extensive research is being conducted to optimize chemotherapy and reduce its side effects (5). Many cancers become resistant to chemotherapy either during treatment or inherently (6). Tumor resistance to anticancer drugs can result from various factors, such as genetic changes in the tumor, genetic differences in the patient, epigenetics, and environmental factors (7). Given these challenges, the use of new methods has always attracted the attention of scientists. One promising source is medicinal herbs, particularly native herbs from different countries. According to the WHO, it is essential to conduct comprehensive testing for the use of medicinal herbs or conventional substances in traditional medicine (5). Therefore, the use of medicinal herbs or certain herbal products during the golden age of medical civilization has been the focus of many scientists, including Avicenna (8).
Chemoprevention is a method of cancer control that uses synthetic or active herbal compounds to prevent cancer progression (9). Pomegranates, scientifically known as Punica granatum L., are members of the Punicaceae family and originate from Iran and its surrounding countries (10). Pomegranates are a natural source of phenolic compounds, tannins, polyphenols, flavonoids, vitamin C, tocopherols, and anthocyanins. The dominant fatty acids in the seeds of most pomegranates include linolenic acid and linoleic acid. Oleic, palmitic, palmitoleic, and caprylic fatty acids have also been identified in various pomegranate varieties. In addition to these fatty acids, pomegranate seeds are rich in steroids, such as gamma tocopherols, alpha-17-estradiol, stigmasterol, beta-sitosterol, and campesterol, as well as non-steroidal compounds, including coumestrol (11, 12). Recent investigations have shown that the seeds and thin, whitish membrane parts of the pomegranate fruit are enriched with a strong antioxidant called punicalagin, which controls superoxide and free radicals, including 1,1-diphenyl-2-picrylhydrazyl (DPPH) (13, 14). Punicalagin inhibits prostaglandin biosynthesis and is cytotoxic to cancer cells (15). Additionally, the polyphenols present in pomegranate seeds act as inhibitors of E2 biosynthesis (17-beta-estradiol), a type of active estrogen, promoting regulated cell death (16). Vitamin D is produced in the skin from 7-dehydrocholesterol by the action of ultraviolet radiation from the sun. It is also present in foods such as enriched milk and fish liver oil (which was first identified in fish liver). Vitamin D is metabolized first in the liver to 25-hydroxyvitamin D, and subsequently in tissues containing 1-alpha hydroxylases, such as the breast, intestine, kidney, and prostate, to 1,25-hydroxyvitamin D, a biologically active hormone. Vitamin D plays a vital role in immune system function, nerve and muscle activity, calcium absorption, bone metabolism, and cellular regulation. Its deficiency can cause irreparable damage, such as the development of breast cancer (17, 18).
2. Objectives
Due to the high prevalence of breast cancer in Iran and worldwide, it is essential to develop new anticancer drugs that do not have cytotoxic effects on normal cells. Therefore, for the first time, the toxic effect of aqueous extract of sweet pomegranate seed (AESPS) on MCF-7 breast cancer and fibroblast cell lines was evaluated and compared with the cytotoxic effect of vitamin D.
3. Methods
3.1. Sample Preparation and Extraction
Sweet pomegranate samples were collected from the Yazd province of Iran. The seeds were dried at room temperature and then ground using a household electric mill. The most appropriate particle size for extraction, according to the extraction standardization guidelines, is between 0.2 and 0.25 mm. A ratio of 1 g of dry plant sample to 10 mL of solvent was used, as this is ideal for extracting active plant compounds (19). To prepare the AESPS, 20 g of seed powder was added to 200 mL of distilled water and subjected to two cycles of distillation by soaking and stirring for 72 hours at room temperature to enhance the dissolution of bioactive compounds in the solvent. After 72 hours, the solution containing the active ingredients was extracted and filtered through Whatman No. 40 filter paper to remove solids. The soluble solvent obtained was separated by rotary evaporation (KIKA*WERKE-), using solvent evaporation at controlled temperature, pressure, vacuum, and repeated rotation of the tank. After drying at room temperature for several days, the residue was stored and sealed in a container.
3.2. Preparation of Different Concentrations of Aqueous Extract of Sweet Pomegranate Seed and Vitamin D
To prepare different dilutions of the extract, 0.008 g of powder was first mixed with 4 mL of RPMI1640 medium (Idezist Co, Iran), resulting in a concentration of 200 mg/mL. This solution was then filtered using a 22 μm syringe filter, and other concentrations of 100, 50, and 25 mg/mL were prepared from it. Vitamin D (1000 IU, Health Aid®) was obtained from the pharmacy and prepared in concentrations of 25, 50, 100, and 200 mg/mL in RPMI1640 medium.
3.3. Cell Culture
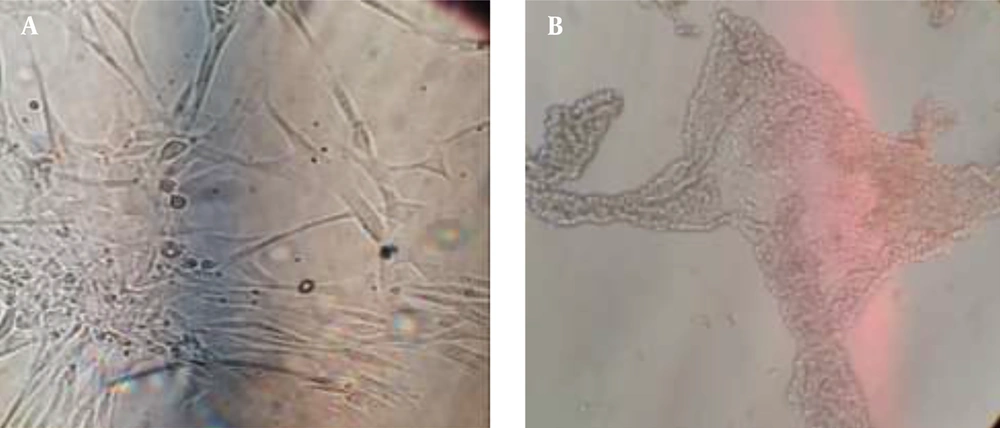
MCF-7 and human fibroblast cells (Figure 1) were purchased from the Pasteur Institute and the Royan Institute (cell banks, Iran), respectively. MCF-7 cells were cultured in DMEM/F12 medium (SIGMA), supplemented with 10% FBS (Bio-Iran Idea) and 1% penstrep (Idezist Co, Iran). Fibroblast cells were cultured in DMEM/F12 medium with 10% FBS (Bioassay), 1% Penstrep, 1% non-essential amino acids, and 1% L-glutamine. The cells were maintained in a CO2 incubator at 37°C with 80 - 90% humidity and a 5% CO2 atmosphere. After three successive passages to reach the desired cell number, the cells were separated using 0.25% Trypsin-EDTA, then centrifuged at 1200 g for 10 - 15 minutes. The supernatant was discarded, and 1 mL of fresh culture medium was added to the pellet to resuspend the cells. Cell counts and viability assays were performed using 0.25% trypan blue. The following formula was used for cell counting:
Then, 10⁴ cells were cultured in 96-well plates. After 24 hours, different concentrations of extract and vitamin D were added to the wells to allow the cells to adhere and recover from the stress induced by trypsinization.
3.4. Methylthiazol Tetrazolium Test
The cytotoxic effects of sweet pomegranate seed extract and vitamin D were tested using the methylthiazol tetrazolium (MTT) assay. Living cells convert the yellow MTT solution into violet crystals, which dissolve in formazan. These crystals are solubilized by dimethyl sulfoxide (DMSO). The percentage of living cells in each well was determined by measuring the absorbance at 570 nm (20). After 24, 48, and 72 hours of cell treatment with different dilutions of sweet pomegranate seed extract and vitamin D, 10 µL of a 5 mg/mL MTT solution was added to each well. The plate was incubated for 4 hours, followed by the addition of 50 μL of DMSO to each well. The plate was incubated for an additional 10 minutes, and then the absorbance was measured using a Nanodrop (BioTek Instruments) at 570 nm. It is worth noting that the test was performed on both MCF-7 cells and human fibroblasts, with three replicates for each. The percentages of cell viability and growth inhibition were determined using the following formulas:
3.5. Statistical Analysis
Since each treatment and control was conducted in triplicate, data were analyzed using Excel 2013 and SPSS version 16 with one-way ANOVA and t-test. The IC50 value was calculated using ed50v10 software. A significance level of less than 0.05 was considered statistically significant.
4. Results
4.1. Effect of Aqueous Extract of Sweet Pomegranate Seed and Vitamin D on the MCF-7 Cell Line
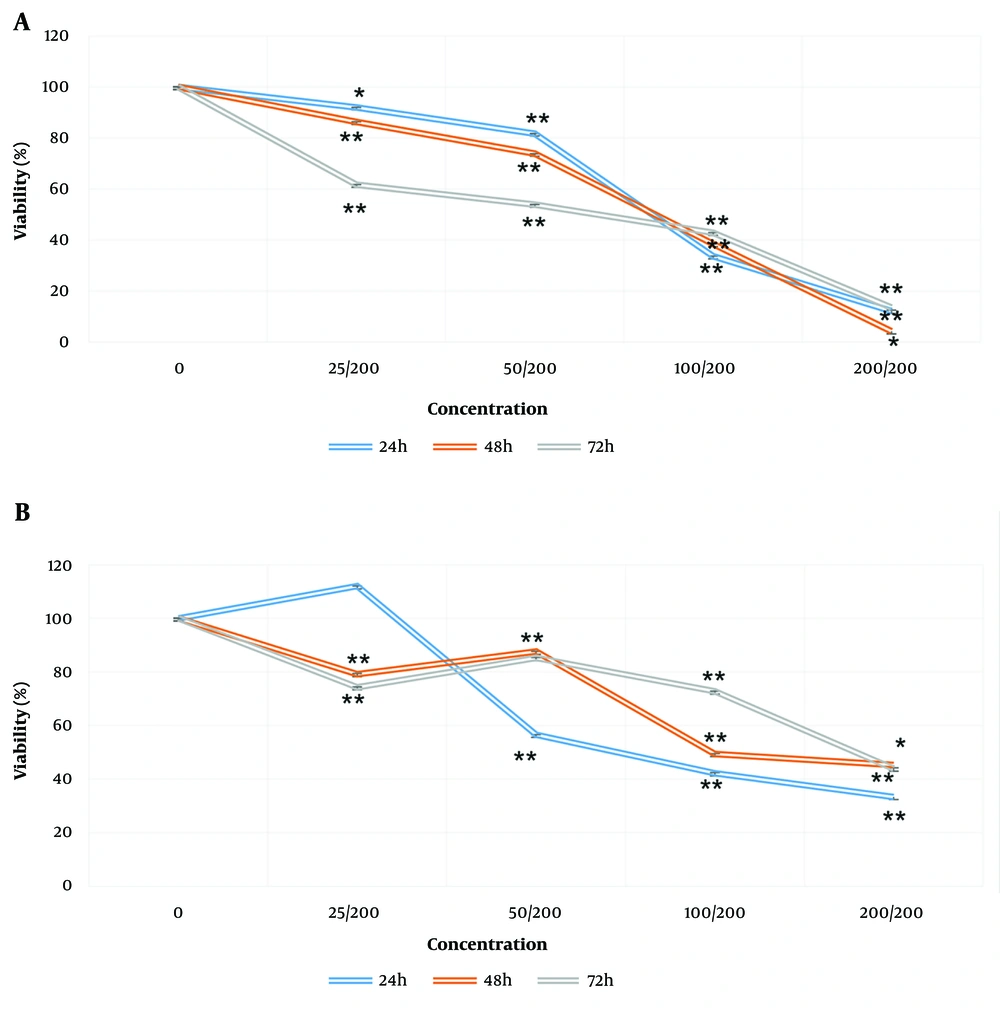
The results showed that a 200 mg/mL concentration of the extract at 24 hours had a significantly higher lethality percentage compared to other concentrations and time points. As the concentration of the extract decreased, the inhibition percentage reduced at 24 hours, with the 25 mg/mL concentration showing no significant difference from the control. At 48 and 72 hours, as the concentration of the extract decreased, inhibition also decreased. Except for the 25 mg/mL concentration, inhibition increased with higher concentrations (Figure 2A). The IC50 increased over time, indicating that longer treatment durations required higher concentrations of the extract to inhibit 50% of cells (Table 1).
| Concentration (mg/mL) | Time (h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Average of Inhibition | IC50 | |||||||
| 24 | 48 | 72 | 24 | 48 | 72 | 24 | 48 | 72 | |
| 200 | 0.181 ± 0.022 | 1.098 ± 0.027 | 1.296 ± 0.1 | 66.63 | 54.75 | 56.06 | 131.68 | 162.26 | 183.99 |
| 100 | 0.213 ± 0.08 | 1.192 ± 0.1 | 2.108 ± 0.08 | 57.83 | 50.62 | 27.22 | |||
| 50 | 0.265 ± 0.01 | 2.07 ± 0.03 | 2.458 ± 0.1 | 43.44 | 12.38 | 14.79 | |||
| 25 | 0.467 ± 0.2 | 1.876 ± 0.1 | 2.151 ± 0.04 | 12.00 | 20.83 | 25.71 | |||
a Significant differences were observed at all concentrations and times except for the 25 mg/mL concentration at 24 hours.
b The significance level was set at 0.05.
c This table reports the concentration in mg/mL and the time in hours.
A, effects of aqueous extract of sweet pomegranate seed (AESPS); and B, vitamin D on the viability of MCF-7 cells. Cells were incubated with increasing concentrations of aqueous extract of sweet pomegranate and vitamin D (25, 50, 100, and 200 mg/mL) in culture medium for 24, 48, and 72 hours. Data are presented as the mean ± SEM of three independent experiments (* P-value < 0.05, ** P-value < 0.001 compared to untreated control cells).
The evaluation of vitamin D on the MCF-7 cell line is presented in Table 2. Results showed that the highest cytotoxic potential was observed at 200 mg/mL at 24, 48, and 72 hours of treatment, with the percentage of cancer cell growth inhibition being approximately the same. In other instances, a decrease in concentration led to a decrease in the percentage of inhibition (Figure 2B). Over time, the IC50 also decreased, meaning that with increasing treatment time, a lower concentration of vitamin D was required to inhibit 50% of cells (Table 2).
| Concentration (mg/mL) | Time (h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Average of Inhibition | IC50 | |||||||
| 24 | 48 | 72 | 24 | 48 | 72 | 24 | 48 | 72 | |
| 200 | 0.227 ± 0.01 | 0.525 ± 0.06 | 1.279 ± 0.07 | 53.98 | 79.71 | 56.69 | 122.05 | 99.41 | 89.87 |
| 100 | 0.220 ± 0.01 | 1.637 ± 0.02 | 2.097 ± 0.08 | 55.82 | 31.25 | 27.62 | |||
| 50 | 0.259 ± 0.02 | 1.365 ± 0.04 | 2.148 ± 0.02 | 45.27 | 43.11 | 25.79 | |||
| 25 | 0.448 ± 0.1 | 1.314 ± 0.005 | 2.042 ± 0.1 | -6.78 | 45.30 | 29.57 | |||
a Significant differences were observed at all concentrations and times.
b The significance level was set at 0.05.
4.2. Effect of Aqueous Extract of Sweet Pomegranate Seed and Vitamin D on Healthy Human Fibroblast Cells
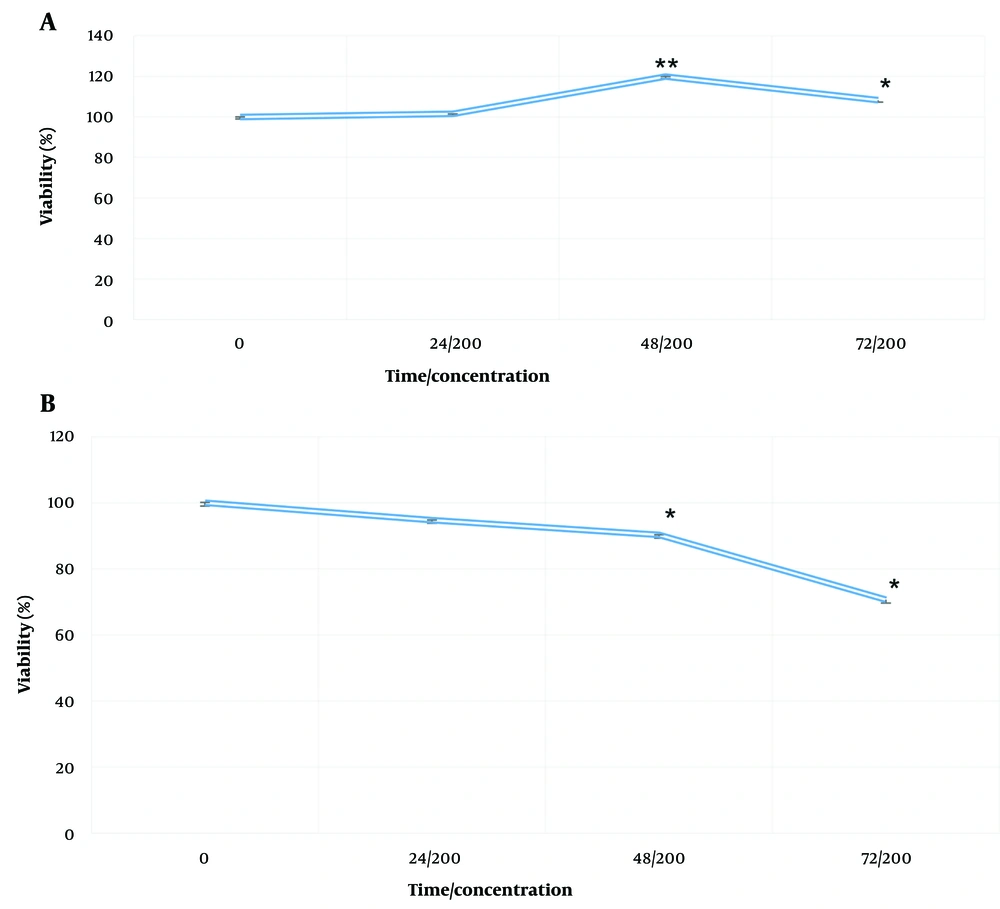
Evaluation of the effect of vitamin D on healthy human fibroblast cells at a concentration of 200 mg/mL at 24, 48, and 72 hours revealed a significant increase in toxicity over time compared to the control group (Figure 3A). The toxic effects of AESPS on healthy human fibroblast cells at 200 mg/mL increased significantly at 24, 48, and 72 hours compared to the control group (Table 3). Healthy human fibroblast cells were not harmed by the AESPSs (Figure 3B).
A, effects of aqueous extract of sweet pomegranate seed (AESPS); and B, vitamin D on the viability of healthy human fibroblast cells. Cells were incubated with a concentration of AESPS and vitamin D (200 mg/mL) in culture medium for 24, 48, and 72 hours. Data are presented as the mean ± SEM of three independent experiments (* P-value < 0.05, ** P-value < 0.001 compared to untreated control cells).
| Groups | Time (h) | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | Average of Inhibition | |||||
| 24 | 48 | 72 | 24 | 48 | 72 | |
| AESPS | 0.362 ± 0.05 | 0.345 ± 0.009 | 0.368 ± 0.02 | -1.49 | -19.90 | -8.23 |
| Vitamin D | 0.338 ± 0.09 | 0.307 ± 0.03 | 0.240 ± 0.01 | 5.22 | 9.70 | 29.31 |
Abbreviation: AESPS, aqueous extract of sweet pomegranate seed.
a A significant difference was observed in all treatments.
b The significance level was set at 0.05.
4.3. Comparison of Aqueous Extract of Sweet Pomegranate Seed and Vitamin D Effect on MCF-7 and Healthy Human Fibroblasts
The results showed that the inhibitory effect of vitamin D was approximately 15% greater than that of AESPS. Aqueous extract of sweet pomegranate seed, however, had no toxic effect on fibroblasts, while vitamin D exhibited 30% toxicity at 72 hours (Tables 1 and 2).
5. Discussion
The study demonstrated that the use of AESPS and vitamin D at concentrations of 25, 50, 100, and 200 mg/mL resulted in cytotoxic effects on the MCF-7 breast cancer cell line (except for 25 mg/mL at 24 hours in the AESPS group). However, vitamin D was approximately 15% more toxic than AESPS. Furthermore, AESPS at a concentration of 200 mg/mL had no cytotoxic effect on the human fibroblast cell line, while vitamin D showed toxicity to human fibroblast cells at the same concentration for 72 hours. Therefore, appropriate doses of AESPS can inhibit the proliferation of MCF-7 cancer cells. Various studies have shown that aqueous extracts possess the lowest antioxidant and highest anti-mutagenic activity due to the presence of phenolic compounds (21, 22). Flavonoids in pomegranate have strong antioxidant properties and, when combined with enzymatic inhibition, can be used as a potential dietary supplement to extend lifespan and prevent cancer (23, 24). Pomegranate's antioxidant activity is attributed to the presence of ascorbic acid (which is separated from active plant compounds during extraction by water), as well as phenolic compounds and anthocyanins (15, 19, 25, 26). Flavonoids and tannins (which are also separated from biologically active constituents during extraction by water) in pomegranate extracts inhibit the growth of cancer cells both in vitro and in vivo (19, 25, 27). The antioxidant property of pomegranate seeds was demonstrated by the reduction of a trivalent iron compound called ferric-tripyridyltriazin, which forms a dual-colored iron complex in the presence of antioxidants, a method known as FRAP (ferric reducing antioxidant power assay). According to the calculation, the FRAP value of pomegranate seeds was 0.72 (12). Polyphenols extracted from pomegranate seeds and their fermented extract have shown inhibition of prostaglandins and cyclooxygenase, preventing breast cancer by suppressing cell proliferation and preventing metastasis in humans (2, 28).
It has also been reported that various parts of pomegranate, including the fruit, juice, seeds, and seed oil, play a significant role in prostate, breast, skin, rectal, lung, and leukemia cancers through an antioxidant mechanism of action. This includes stopping growth factors, disrupting the cell cycle, promoting apoptosis, anti-angiogenesis, and exerting anti-inflammatory effects (2, 29, 30). Pomegranate seeds contain various fatty acids, such as punicic acid, leucic acid, palmitic acid, stearic acid, linolenic acid, and tocopherols, as well as vitamin C, phenols, sterols, and sexual steroids (26, 31). Vitamin D intake is inversely related to breast cancer risk, as revealed by studies on breast cancer with estrogen receptor (ER) and progesterone receptor (PR) status. By taking this vitamin, anti-cancer mechanisms are activated through 17-beta-estradiol and down-regulation of ERs. This suppresses the growth and development of cancer cells in the G1 to S phase, thereby decreasing the risk of developing breast cancer (32-34).
5.1. Conclusions
In summary, the lethal effect of AESPS on MCF-7 cancer cells was confirmed, while its impact on human fibroblast cells was not verified. This extract has the potential to be further studied in the synthesis of chemotherapeutic drugs after additional laboratory and clinical studies. Additionally, taking vitamin D supplements or consuming foods rich in this vitamin may help prevent breast cancer, though further clinical studies are needed.