1. Context
Regenerative medicine (RM), which emerged in the early 20th century, has transformed from foundational studies on regeneration and development into the forefront of modern cellular therapies (1). Initially rooted in ancient methods of promoting tissue healing, the field now employs sophisticated techniques aimed at restoring natural tissue function in diseased or damaged areas (2). By leveraging advancements in stem cell technology and tissue engineering, RM offers groundbreaking solutions for acute injuries, chronic diseases, and congenital abnormalities (3). The field's evolution is marked by significant milestones, including breakthroughs in transplantation research during the 20th century, and a more recent focus on translational medicine, which seeks to bridge laboratory discoveries with clinical applications (1). Its progress has been shaped by a convergence of commercial, technical, and socioeconomic factors, with recent innovations indicating that RM is approaching a critical phase in its development (4). Emerging cellular therapies and tissue-engineering approaches are poised to replace conventional treatments for joint and bone conditions (2).
Regenerative medicine's potential lies in its ability to revolutionize therapeutic strategies through innovative technologies and pioneering research (5). Central to this progress is ongoing work in stem cell technologies, which underpin diverse methods for tissue and organ regeneration (6). Recent advancements in bioengineering—including mechanobiology, biomaterials, intracellular delivery systems, and computational modeling—have further accelerated progress in the field. Notably, nanotechnology, such as using magnetic nanoparticles that mimic biological structures, has become a powerful tool for enhancing regenerative capabilities. Together, these innovations are breaking new ground, addressing translational challenges, and paving the way for personalized and precise treatments (7-9).
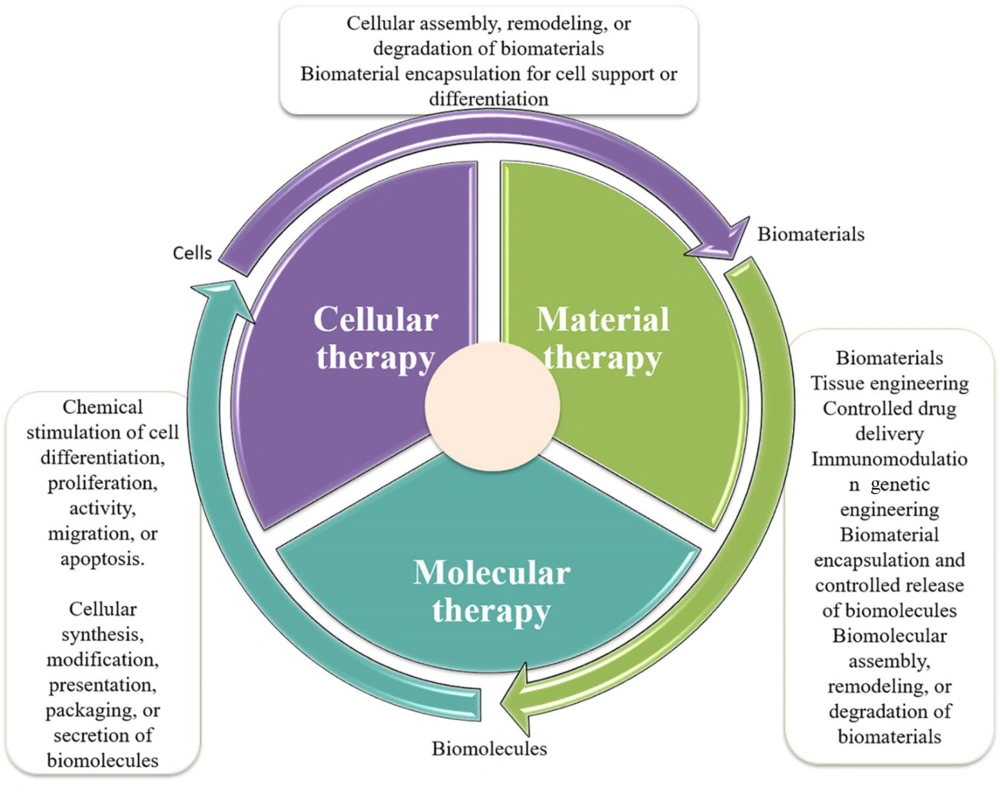
The breadth of RM has expanded significantly with the advent of novel platforms, including tissue engineering, gene editing, and cell sheet technology (Figure 1) (10, 11). These advancements promise to redefine healthcare by offering personalized treatment options beyond symptom management, aiming instead to provide lasting solutions (12). Healthcare systems are expected to experience profound benefits, including improved patient outcomes, enhanced quality of life, and potential cost savings from reduced reliance on chronic care (13). Additionally, new imaging techniques now enable real-time monitoring of responses to regenerative therapies in live subjects, overcoming the limitations of post-mortem evaluations and traditional monitoring methods. This capability facilitates more effective evaluation of treatment outcomes and allows for timely adjustments to therapeutic strategies (14, 15).
Three approaches to regenerative medicine (16).
2. Breakthroughs and Key Developments in Regenerative Medicine
2.1. Stem Cells and Organoids
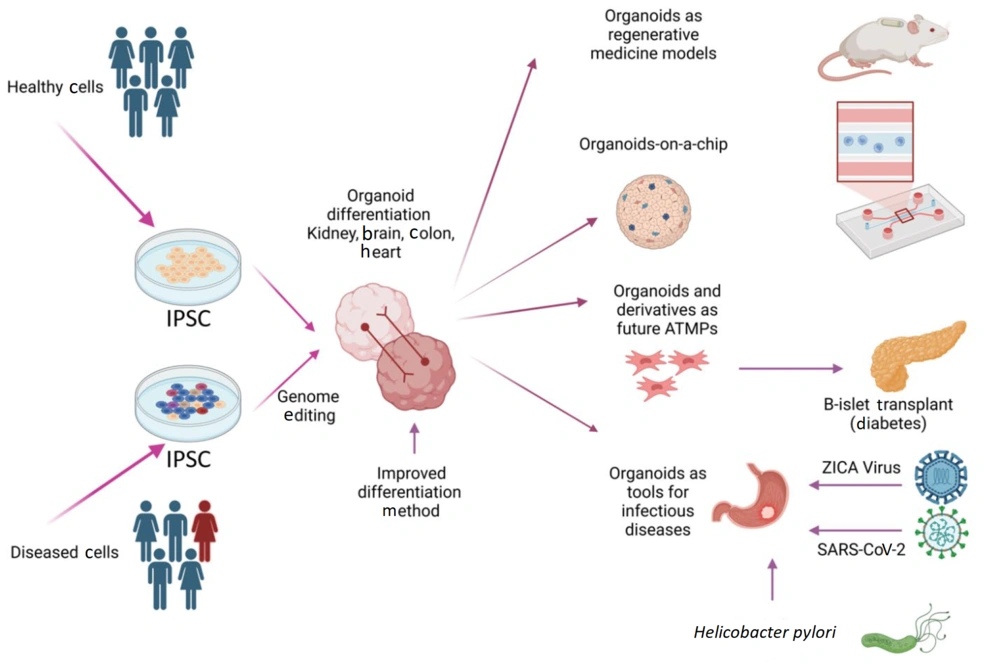
Induced pluripotent stem cells (iPSCs) are increasingly proving transformative for disease modeling, drug screening, and RM (Figure 2). By utilizing human pluripotent stem cells (hPSCs), researchers can generate organoids—3D miniaturized versions of organs that mimic the structure, function, and developmental processes of their full-sized counterparts (10). Notable advancements have been achieved in generating organoids for the human brain, heart, liver, and kidneys (17). Additionally, organs such as the liver, pancreas, and thyroid can now be rapidly and efficiently derived from the anterior definitive endoderm stage through marker-based cell line selection (18-20). These advancements have mitigated the challenges associated with whole-organ culture, paving the way for more effective personalized medicine and drug screening.
Induced pluripotent stem cells (iPSCs), -derived organoids and their potential use in regenerative medicine (21).
In parallel, researchers have identified molecularly defined factors that enable the immortalization of tissue-resident stem cells, significantly enhancing their utility. For instance, human umbilical vein endothelial cells (HUVECs) derived from peripheral blood and human dermal fibroblasts can be immortalized through the overexpression of specific phenotypes (22).
The integration of organoids into microfluidic (MF) devices has further revolutionized the field. These "organs-on-a-chip" systems replicate the in vivo environment of specific organs, fulfilling critical engineering requirements for modeling physical, chemical, and biological interactions (23). Since an organ comprises multiple interacting components, MF devices allow researchers to emulate these interactions, standardizing and improving the systems' accuracy (24). Such technologies promise to advance drug discovery, providing physiological data that could ultimately reduce the reliance on animal experiments.
In the context of patient-specific RM, the field continues to make strides in improving the safety and efficiency of iPSCs. These cells have already shown promising results in the treatment of Parkinson’s disease (PD), Alzheimer’s disease (AD), and cardiovascular conditions (25). Meanwhile, mesenchymal stem cells (MSCs), derived from sources such as bone marrow, adipose tissue, and neuronal tissue, offer another promising avenue. Importantly, MSCs are less likely to trigger immune rejection, further underscoring their therapeutic potential (26).
Mesenchymal stem cells have shown potential in suppressing T-cell proliferation and modulating immune responses, thereby playing a critical role in reducing organ rejection and alleviating symptoms of autoimmune diseases (27, 28). Furthermore, exosomes—small vesicles containing proteins, lipids, mRNA, and microRNA—derived from MSCs exhibit regenerative properties that address some limitations of traditional cellular therapies, such as immune rejection and tumorigenesis (29, 30). Exosomes also offer scalable, stable, and safe therapeutic effects, particularly in tissue repair.
The rapid growth in clinical trials for stem cell therapies highlights their expanding applications, particularly in cardiovascular and neurodegenerative diseases (31, 32). For example, iPSCs are being extensively studied for neurodegenerative diseases like Parkinson’s disease, where they demonstrate promising effects on neuronal repair and inflammation reduction (33). Hematopoietic stem cells (HSCs) remain the most extensively studied stem cells in clinical trials, focusing on leukemia and inherited blood disorders (34). However, ensuring the standardization of effective and reproducible protocols and prioritizing patient safety remains paramount in RM research.
2.2. Advances in Gene Therapy
Regenerative medicine is leveraging cutting-edge tools such as CRISPR-Cas9 gene-editing technology and synthetic mRNA to optimize the effectiveness of cell and gene therapies (35). Among these, CRISPR-Cas9 stands out as a revolutionary technology, enabling precise removal of disease-causing mutations in stem cells. These modified cells can then be transplanted back into patients to restore healthy function (36). The application of gene editing to HSCs has demonstrated significant potential for treating blood disorders and is steadily advancing toward clinical trials (37). Efforts to refine CRISPR-Cas9 have successfully reduced off-target effects, enhancing its safety and feasibility for clinical use (38).
In addition to gene editing, synthetic mRNA therapy has emerged as a powerful tool for influencing cellular behavior, resulting in enhanced tissue regeneration. This approach has shown promising outcomes in repairing heart and spinal cord tissues (39). Looking ahead, the integration of gene-edited stem cells into RM models is expected to revolutionize treatment strategies, expanding the potential for regenerative cell therapy to address chronic diseases such as muscular dystrophy and cystic fibrosis (40).
2.3. 3D Bioprinting and Scaffold Technology
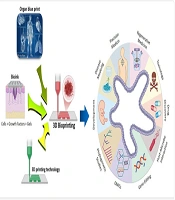
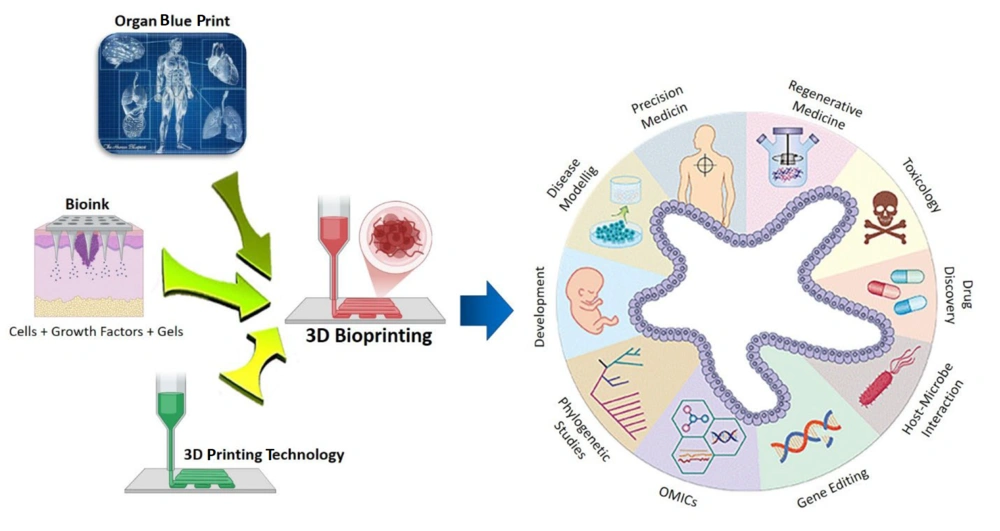
The development of three-dimensional (3D) bioprinting technology, in conjunction with scaffold-based regeneration platforms, has significantly advanced the field of RM (Figure 3) (41). Using bio-inks infused with live cells, 3D bioprinting enables the precise construction of microscale structures that mimic the architecture of target tissues, making them suitable for both research and transplantation purposes (42, 43).
3D bioprinting integrates conventional 3D printing, imaging, and cell-gel to fabricate functional tissue for regenerative medicine (RM), pharmaceutical preclinical drug screening, and animal-free meat (44).
In the short to medium term, evaluating the biological functions of regenerated tissues will be essential. Achieving rapid vascularization and tissue remodeling is a key focus, likely facilitated by the intricate hierarchical patterns and spatial complexity of artificial tissues. These advancements are further enhanced by the integration of cutting-edge scaffold materials (45, 46). Decellularized extracellular matrix (dECM)-based scaffolds have emerged as promising tools for creating mechanically robust and biocompatible tissue patches, particularly in cardiac and orthopedic applications (47, 48).
The incorporation of scaffolding materials also accelerates the use of biodegradable platforms for in-situ growth factor delivery, promoting cell survival, differentiation, and tissue regeneration (49, 50).
2.4. Neurodegeneration and the Role of Soft Robotics
Regenerative medicine presents significant opportunities for addressing neurodegenerative diseases by enhancing the body's inherent self-repair mechanisms (51). Various strategies, including stem cell therapy, gene therapy, and nanomedicine, have been developed to combat conditions such as Alzheimer’s, Parkinson’s, and Amyotrophic Lateral Sclerosis (52). These interventions primarily leverage neural stem cells, which possess the intrinsic capacity for self-renewal and the ability to differentiate into diverse glial and neuronal cell types (53).
When integrated with biomaterials and tissue engineering, these approaches can regenerate damaged nerves and help preserve healthy neurons and glia (54). Advances in biomaterials, such as electrically conductive hydrogels, have provided critical tools for neuro-regeneration. These hydrogels act as "molecular glue," creating essential connections for nerve repair and facilitating the transmission of electrical impulses between neurons and nerves (55, 56).
Soft robotics, an emerging and transformative field, also holds promise for treating severe nerve injuries by enabling the development of brain-controlled prosthetics and advanced spinal cord implants (57). By utilizing compliant and adaptable materials, soft robotics provides innovative solutions for medical applications, including prosthetics, drug delivery, and minimally invasive surgical tools (58, 59). In RM, the combination of soft robotics with stem cell technologies enhances tissue regeneration, offering a flexible platform to support complex therapeutic functions (59). The integration of living cells into soft robotic systems enables dynamic responses to external stimuli, such as sensing and actuation, unlocking new possibilities for healthcare applications (59, 60).
Additionally, bioelectric circuits are emerging as a powerful technology to accelerate cellular repair processes, offering potential solutions for treating metabolic and neurological disorders (61). The fusion of bioelectric circuits with regenerative scaffolds represents a significant step forward in repairing nerve tissue and advancing the broader field of tissue regeneration.
3. Organs-on-a-Chip and Micro-Physiological Systems
Organ-on-a-chip technology represents a groundbreaking advancement in micro-engineered biomimetic systems, designed to replicate the structural and functional characteristics of human tissues and organs (62). These platforms typically comprise microfluidic channels, cell culture chambers, and stimuli sources, all integrated into oxygen-permeable, transparent materials (63). By mimicking human organ physiology and function, organs-on-a-chip (OOAC) devices provide controlled environments for studying disease mechanisms, testing drugs, and investigating organ interactions (64). Innovations such as liver-on-a-chip, heart-on-a-chip, and multi-organ "body-on-a-chip" systems are transforming drug safety and efficacy assessments, significantly reducing the reliance on animal models (65). These chips allow researchers to recreate disease environments, enabling the testing of new drugs in conditions closely resembling those inside the human body. This approach not only accelerates the translation of laboratory research into clinical applications but also enhances the precision and relevance of experimental outcomes (66).
4. Artificial Intelligence and Nanotechnology in Regenerative Medicine
Artificial intelligence (AI) has introduced transformative advancements to RM, automating iPSC research and enhancing the applicability of technologies for disease modeling and drug discovery. Machine learning (ML) and deep learning (DL) methods, particularly convolutional neural networks (CNN) and support vector machines (SVM), have been developed to automate the identification and classification of iPSC-derived cells (67, 68). Artificial intelligence systems consistently outperform humans, avoiding judgment subjectivity and reducing errors, which is crucial for the clinical-scale production of human cells (69). Artificial intelligence also leverages big data analytics to identify patterns such as heterogeneity and drug response in iPSC lines derived from diverse genetic backgrounds. This capability allows AI to integrate data and predict potential drugs' qualitative and quantitative effects (70).
Artificial intelligence-guided solutions like DeepNEU and PhenoTox have revolutionized high-throughput, label-free drug screening. These tools enable rapid analysis of drug efficacy and toxicity in iPSC-derived tissues. For example, PhenoTox uses deep learning methods to detect early cellular changes indicative of toxicity—changes that might take a pathologist an average of 15 months to identify. By identifying toxic effects earlier than the human eye can detect them, PhenoTox helps mitigate side effects and enhances the system’s ability to learn and predict induced toxicity (71). During crises like the COVID-19 pandemic, the integration of AI and iPSCs proved invaluable. Artificial intelligence was employed to study the virus's effects on lung cells, uncovering cellular vulnerabilities to COVID-19 infection. This synergistic approach between AI and iPSC technology has demonstrated immense potential to accelerate drug development and regenerative therapies, paving the way for groundbreaking innovations in RM (72).
Nanotechnology plays a pivotal role in RM by advancing the design of nanoparticles for improved drug delivery and stem cell biodynamics. Nanostructured surfaces with biomimetic properties are engineered to integrate seamlessly with host tissues and support cell growth (73). In aesthetic medicine, personalized RM techniques are employed for facial and neck rejuvenation, leveraging nanotechnology for precise interventions (74). Nanocarriers are utilized to transport cell growth factors and protective agents directly to target cells, facilitating tissue repair and anti-aging at the nanoscale (75). Light-triggered nanostructures offer additional therapeutic capabilities, delivering reagents to tissues to rejuvenate cells and prevent damage. The integration of nanotechnology with RM applications—such as plastic surgery, beauty injections, autologous fat transfer, and platelet-rich plasma (PRP)—has enabled personalized and effective treatments for both medical and aesthetic needs (76, 77).
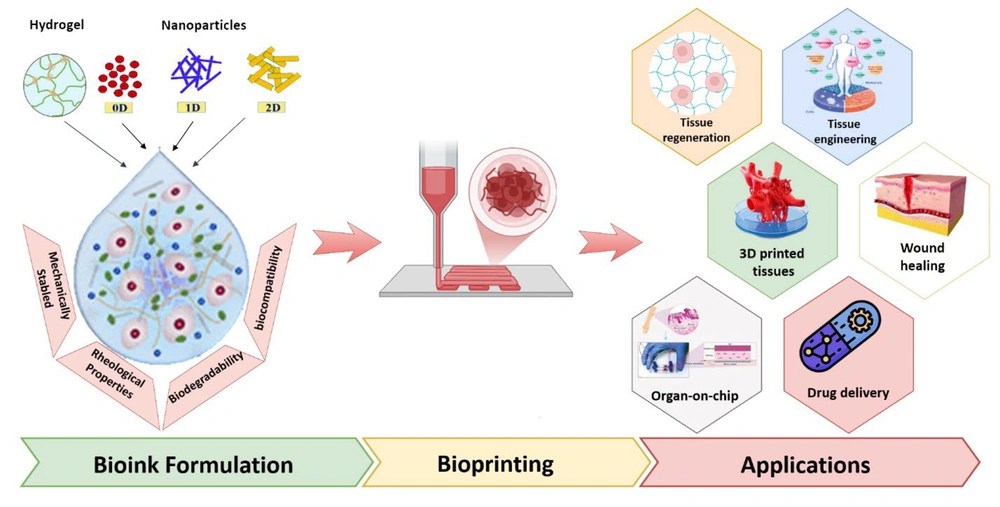
In the realm of tissue engineering, nanotechnology has driven advancements in bioinks for 3D bioprinting. These bioinks require enhanced bioinspired rheological and mechanical properties to replicate soft tissues like kidneys, hearts, and other organs. Hybrid bioinks infused with nanomaterials offer promising solutions to these challenges and are gaining significant attention from researchers. Both natural and synthetic nanomaterials, including carbon nanotubes, graphene oxides, titanium oxides, nanosilicates, nanoclay, and nanocellulose, are being incorporated into 3D bioprinting processes. These materials improve bioprintability, biocompatibility, and biodegradability, addressing critical requirements for successful tissue engineering (Figure 4) (78).
Nanomaterials-based hybrid Bioink Platforms in advancing 3D bioprinting technologies for regenerative medicine (RM) (78)
5. Conductive Hydrogels and Nanorobotics
The integration of nanorobots with conductive hydrogels represents a groundbreaking advancement in RM, introducing innovative approaches for tissue repair and regeneration (79). Conductive hydrogels are designed to modulate local electric microenvironments across neural tissues, bridging gaps and enhancing cellular communication during nerve repair. These hydrogels can transduce bioelectric signals, effectively mimicking endogenous electrical channels that conduct natural electricity (80, 81). These bioelectric signals are critical for nerve function, playing a key role in transmitting regenerative cues and restoring damaged nerve pathways (82).
Nanorobots, or nanoscale robots, add another dimension to this field. They are anticipated to enable cellular and micro-level operations, including the precise manipulation of nanostructured materials, targeted drug delivery, and tissue treatment without adverse side effects (83, 84). These capabilities open up new possibilities for precise and effective regenerative therapies.
In addition to hydrogels and nanorobots, robotic systems combining these technologies are paving the way for biomechanical artificial organs and prostheses. These devices are designed to replicate natural movements and physiological functions, representing a transformative innovation in RM (60, 85).
6. Ultrasound-Based Extracellular Matrix Bioengineering
Recent advancements in technology have highlighted the pivotal role of acoustic fields, particularly ultrasound (US), in advancing the manufacturing of extracellular matrix (ECM)-based biomaterials (86). Ultrasound techniques enable the spatial organization and molecular cross-linking of ECM materials through thermal and mechanical effects, facilitating the creation of both macro- and nano-scale architectures for scaffold design in tissue engineering (87, 88). By selectively influencing ECM proteins, such as collagen, US can produce highly organized and biomimetic structures that support essential cellular behaviors for tissue repair and regeneration. These engineered scaffolds exhibit enhanced mechanical robustness and superior biological compatibility, critical for successful integration with host tissues (89, 90).
Furthermore, US-driven manipulation of ECM structures can guide cellular orientation and morphology during scaffold polymerization. By altering collagen fiber density and ECM architecture, US enables the fabrication of biomimetic tissues that closely resemble the native cellular environment. This capability makes US an invaluable tool for engineering vascularized tissues and organoids, significantly enhancing their therapeutic potential (91, 92).
7. The Future of Healing: Next-Generation Regenerative Solutions
Regenerative medicine is revolutionizing the future of healing by introducing innovative methods and strategies for tissue repair and reconstruction (93). Duscher (2015) highlighted the vast potential of stem cells in wound healing, positioning them as an optimal choice for clinical applications (94). Similarly, Anitua (2010, 2013) championed the use of endogenous regenerative technologies, leveraging a patient’s own proteins and growth factors to enhance tissue and bone generation (95). A prominent example is PRP, which has demonstrated remarkable results in clinical settings (96). Julier (2017) expanded the scope of regenerative medicine by emphasizing the regulation of immune responses as a promising strategy to create a pro-regenerative environment within injured tissues (97).
These advancements have been successfully translated into clinical practice, as evidenced by the adoption of synthetic bone graft substitutes (98) and licensed artificial skin technologies like ReCell, which have proven effective beyond laboratory trials in real-world scenarios (99). Technological progress in skin RM has led to treatments that not only heal wounds but also improve their functionality (100). The introduction of bioactive dressings, such as hydrogels, alginates, and hydrofibers, has been transformative. These dressings maintain optimal moisture levels for chronic wound management while simultaneously promoting cellular activity (101). Moreover, incorporating growth factors and anti-inflammatory agents into these materials has expanded their utility, advancing from temporary solutions to more comprehensive therapeutic approaches (102).
Autologous fibroblast and keratinocyte-based engineered skin substitutes have further enhanced epidermal regeneration by acting as signaling conduits (103). Collectively, these advancements represent a milestone in personalized medicine, heralding a new era in 21st-century medical innovation (104).
8. Challenges and Policy Implications
The clinical translation of RM faces significant challenges, including the identification of optimal cell sources, the development of suitable biomaterials, and the establishment of reliable methods for cell expansion and three-dimensional culture (105). Cell sheet engineering, a scaffold-free approach that has shown positive clinical outcomes, still encounters obstacles in achieving industrial scalability and widespread adoption (106). Mesenchymal stem cells are particularly promising for clinical applications due to their self-renewal capacity, multilineage differentiation potential, and immunomodulatory properties (107). However, translating basic research into effective therapies demands multidisciplinary collaboration and rigorous attention to safety concerns associated with cell- and tissue-based products (108). Issues such as rapid cell migration from target sites, tumorigenicity risks, and high costs continue to limit the broader application of these technologies (109).
Long-term safety and efficacy remain critical areas for investigation, necessitating well-structured clinical trials (110). Additionally, replicating the complex biological environments of human organs in engineered tissues is an ongoing challenge (111). Expanding research to include larger sample sizes and conducting comprehensive long-term evaluations will be essential for overcoming these barriers and advancing regenerative technologies into routine clinical practice (112).
Regenerative medicine presents complex policy challenges that require innovative approaches to stakeholder engagement and regulation. These "wicked policy issues" involve a diverse array of stakeholders with competing interests, highlighting the need for effective public education and engagement strategies (113). The field also grapples with ethical, legal, and social implications, necessitating comprehensive efforts in capacity building, policy development, industry collaboration, research ethics, communication, and community engagement (114). The development of new regulatory regimes for advanced therapies poses the risk of stifling innovation if they simply extend existing frameworks without accommodating the unique aspects of RM (115).
To address these concerns, a "responsible research and innovation" (RRI) approach has been proposed. This approach integrates insights from science and technology studies to facilitate the responsible acceleration of RM. It seeks to balance the urgency for rapid access to cell therapies with the imperative for readiness, safety, and value creation in the field (116). Furthermore, establishing uniform standards is essential to protect patients from the risks associated with unproven stem cell interventions while supporting the advancement of promising RM products (117).
9. Case Studies: Mayo Clinic's Model
9.1. Mayo Clinic's Integration of Regenerative Medicine
The Mayo Clinic serves as a leading example of the successful integration of RM into clinical practice. Their model includes:
9.2. Discovery-Translation-Application Framework
The Mayo Clinic has developed a structured approach that spans from initial research through to clinical application, ensuring that new therapies are rigorously tested and validated before being offered to patients (118).
9.3. Patient-Centered Care
Emphasizing a patient-centered model, the clinic integrates regenerative therapies within existing medical specialties, facilitating access and continuity of care (118, 119).
9.4. Clinical Trials as Catalysts
Ongoing clinical trials play a critical role in assessing new regenerative treatments, providing valuable data on their effectiveness, and guiding future applications (119).
10. Conclusions
Regenerative medicine represents a promising and diverse interdisciplinary platform with the potential to shift the focus of healthcare from symptom-based treatment to achieving true restoration of health. With advancements in stem cell technology, gene editing, and bioengineering, the prospects for personalized treatments and the reconstruction of tissues and organs have reached unprecedented heights. Innovations such as 3D bioprinting, organoids, and cutting-edge scaffolding techniques are already turning some long-held aspirations into reality, addressing conditions once deemed untreatable.
However, significant challenges remain on the path to widespread clinical translation. Key barriers include ensuring patient safety, navigating ethical and regulatory complexities, and addressing logistical concerns, all of which are critical for achieving public acceptance of these transformative technologies. To fully realize the revolutionary potential of regenerative medicine, closer collaboration between researchers, clinicians, and policymakers is essential. This interdisciplinary synergy will be instrumental in overcoming the existing hurdles and paving the way for a new era of medical therapies. By doing so, RM can profoundly reshape the future of healthcare, offering innovative solutions for both physical and mental health restoration.