1. Background
Lung cancer is among the most commonly diagnosed cancers globally and is the leading cause of cancer-related deaths. In 2012, approximately 1.6 million people died from lung cancer, and the number of deaths from this disease is projected to rise to 3.1 million by 2035 (1, 2). Therefore, finding effective treatments is one of humanity’s significant challenges (3, 4). Lung cancer prognosis and survival rates remain low due to late diagnosis, medication resistance, and side effects, despite major advancements in diagnostic and therapeutic approaches. Consequently, new therapeutic approaches, such as the use of natural substances that are less harmful, have garnered interest in the treatment of lung cancer.
Turmeric (Curcuma longa), a perennial herbaceous plant belonging to the ginger family (Zingiberaceae), is often referred to as Curcuma domestica (5). The medicinal properties of curcumin have been extensively researched in recent years. This substance can penetrate the blood-brain barrier and possesses strong neuroprotective and anti-inflammatory properties. Curcumin has demonstrated anticancer effects in lung cancer through several mechanisms, including inhibition of the cell cycle and proliferation, invasion and metastasis, activation of apoptosis, epigenetic modifications, and regulation of different gene expressions (6).
Although curcumin has favorable pharmacokinetic profiles and is inherently safe, its efficacy is limited by poor solubility and instability in aqueous environments, leading to a short half-life and rapid clearance from the bloodstream. Nanoparticle-based drug delivery methods, which load curcumin into solid lipid microparticles such as bovine serum albumin or liposomes, address issues of poor solubility and low bioavailability. Evidence from various publications indicates that curcumin nanostructures exhibit superior solubility and stability (7, 8). Unlike free curcumin, nano-curcumin dissolves more quickly in tissues and plasma. In an in vivo study in mice, Bhawana et al. demonstrated that nano-curcumin enhanced tissue dispersion and biocompatibility by providing a biological half-life 60 times longer than that of native curcumin therapy (9). In this investigation, we reduced the curcumin particle size to 2 - 40 nm using a milling process, discovering that this technique produces nano-curcumin with good chemical and physical stability (10).
Different cell cycle regulatory mechanisms have been conserved throughout evolution and regulate cell division. Cell cycle checkpoints serve as DNA monitoring systems that prevent the accumulation and propagation of genetic errors during cell division. The unchecked development of cancer cells is caused by cancer-related mutations that disrupt cell cycle regulation. These dependencies can be best utilized in cancer treatment, according to new studies on cell cycle regulatory mechanisms and their role in cancer (11). In some cases, cell cycle defects result from loss-of-function mutations of p53, arguably the most common genetic defect in cancer (12). TP53, known as the guardian of the genome, responds to various intrinsic and extrinsic stressors, including DNA damage, oncogene activation, nutrient deprivation, and hypoxia (13, 14). The TP53 gene is involved in a growing number of biological processes, such as metabolism, senescence, autophagy, apoptosis, cell cycle arrest, and DNA repair. TP53 gene instability is a key factor in lung cancer development and is crucial in malignancy in the epithelial cells of the lungs. Enhancing the survival rate of patients with lung cancer may require a more positive treatment approach based on a better understanding of the role of TP53 in lung carcinogenesis (15).
2. Objectives
The present study aimed to evaluate the effect of nano-curcumin, without any carrier or delivery system, on cell cycle inhibition through its impact on p53 gene expression in lung cancer cell lines for the first time. We found that nano-curcumin can inhibit the cell cycle in a targeted manner by increasing p53 expression and inducing cell death in cancer cells. Therefore, this study provides evidence that nano-curcumin has the potential to inhibit lung cancer proliferation and progression in a targeted manner.
3. Methods
3.1. Synthesis of Nano-Curcumin
3.1.1. Conversion of Curcumin to Nano-Curcumin
In this study, the curcumin compound (CURN) was synthesized in the Department of Chemistry laboratory at Shahid Chamran University of Ahvaz by modifying the method described by Bhawana et al. (9). For the formation of the organic phase, 125 mg of curcumin was dissolved in 25 mL of dichloromethane. The aqueous phase was prepared by mixing 90 mL of boiling water with 10 mL of 5% (v/v) Triton X-100 solution. A magnetic stirrer was used to stir the contents at 1500 rpm for 20 minutes at room temperature until a yellow color appeared. Next, 2 mL of the organic phase was added dropwise to the aqueous phase (approximately 10 drops/min) under ultrasonic conditions, using an ultrasonic power of 100 W and a sound frequency of 40 kHz. This conversion was confirmed by measuring the particles using DLS, TEM images, and UV-visible spectra (16).
3.2. A549 Cell Line Culture and Viability Assessment
The A549 cell line, obtained from the Pasteur Institute of Tehran, was cultured at 37°C with 5% CO2 and 95% humidity in RPMI media containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cell viability was assessed using trypan blue staining and Neobar slide counting by conventional procedures (17).
3.3. Cell Treatment and IC50 Analysis
After 24 hours of culture, nano-curcumin at concentrations of 1.5, 3, 4.5, and 6 μM was added to the treated groups, while the control group received only culture medium. After 48 hours, an MTT assay was performed on 96-well plates, with DMSO used as the solvent, and absorption measured at 570 nm (18).
3.4. Flow Cytometry
A549 cells were cultured in control and treated media for 48 hours, harvested with 0.25% trypsin/EDTA, and fixed with 2% formaldehyde. Cells were stained with FITC-conjugated monoclonal antibodies targeting SH3, STRO-1, and other antigens. Background staining was assessed using PE-conjugated nonspecific IgG (19, 20).
3.5. RNA Extraction
In this study, A549 cells were cultured in six-well plates and exposed to IC50 concentrations of nano-curcumin for 48 hours. RNA extraction was performed using an RNX solution. Following treatment, the medium was removed, and the wells were washed with PBS. RNX solution (400 μL) was added, and samples were centrifuged after chloroform addition to separate phases. The supernatant was mixed with RNA-isopropanol, centrifuged, and rinsed with 75% alcohol. The RNA pellet was dissolved in 40 μL DEPC water and stored at -70°C (21).
3.6. cDNA Synthesis
Random hexamer primers and oligo dT were used for RNA amplification. A kit from Sina Clone was used for cDNA synthesis. Initially, RNA, dNTPs, and primers were added to a 0.2 μm tube, and the final volume was adjusted to 10 μL using DEPC water; it was heated for 5 minutes at 70°C using a thermocycler to open the secondary structures of RNA. Then, according to the kit protocol, other materials, including enzyme, buffer, and RNase inhibitor, were added to each tube, and the final reaction volume was adjusted to 20 μL. It was then incubated at 45°C for 50 minutes in a thermocycler, and finally, it was placed at 85°C for 5 minutes to stop cDNA synthesis (inactivate the RT enzyme) (22).
3.7. Real-time PCR
Primers were designed using Sinaclone Iran’s Primer3. RT-PCR was performed using ABI (AB application Biosystem) and SYBR Green solution (Amplicon). The expression of p53 was monitored using GAPDH as an internal control. The reaction was performed using a real-time PCR machine, with two replicates considered for each sample in each cycle (23).
3.8. Western Blot
Western blot was carried out per standard protocols (avinstemgene):
(1) A 50 mM Tris base lysis buffer (pH 7.4) was prepared by dissolving 0.3 g of Tris base in water and bringing the volume to 50 mL; (2) tissue samples, stored frozen, were centrifuged (14,000 rpm, 10 minutes), and the supernatant was stored at -70°C after Bradford protein quantification; (3) for SDS-PAGE, samples were mixed 1:1 with 2X SDS loading buffer, boiled (5 minutes), and briefly centrifuged and (4) protein concentrations were measured by the Bradford method using BSA as a standard (24).
3.9. Statistical Analysis
Real-time PCR data were analyzed using the Livak method, and statistical analysis was performed using GraphPad Prism 9.5 with one-way ANOVA. Results were deemed significant when the P-value was less than 0.05.
4. Results
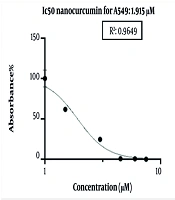
Nano-curcumin can reduce the viability of lung cancer cell lines. The cells were treated with 1.5, 3, 4.5, and 6 micromolar (μM) concentrations of nano-curcumin in 96-well plates, in triplicate, for 48 hours for the MTT assay. The results of the ELISA were analyzed to obtain the IC50 using GraphPad Prism 9 software, and a graph was generated as follows. The IC50 value of nano-curcumin for A549 cells at 48 hours was determined to be 1.915 μM (Figure 1).
4.1. Nano-Curcumin Induces Cell Cycle Arrest
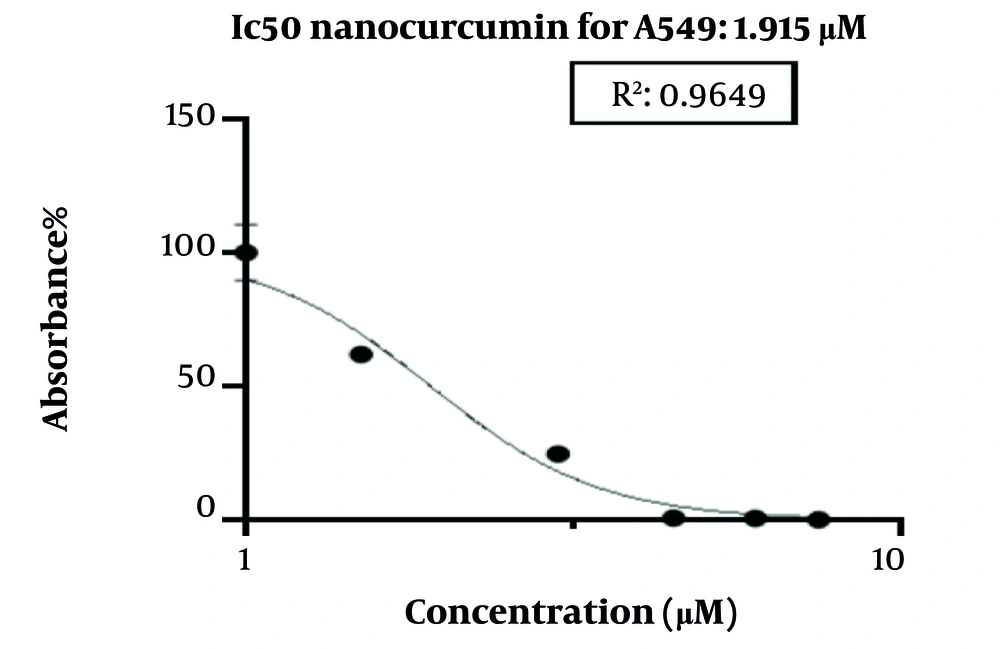
To understand the fundamental mechanisms of curcumin’s antiproliferative action, we evaluated the cell cycle in cells treated with nano-curcumin using flow cytometry. As shown in Figure 2, curcumin at the IC50 concentration (1.915 μM) induced cell cycle arrest in cancerous cells. The findings indicated that nano-curcumin could induce cell cycle arrest in the G1/S phase.
4.2. Increase in p53 Expression Levels in Cells Treated with Nano-Curcumin
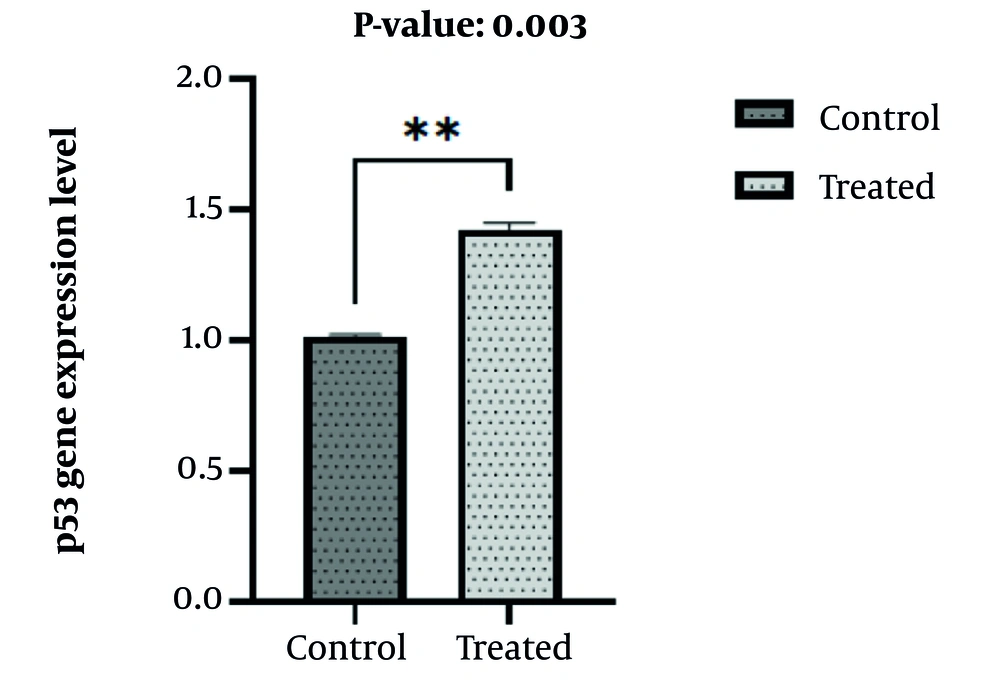
Results from real-time PCR revealed an increase in p53 expression in nano-curcumin-treated samples (IC50) compared to the control group after 48 hours (Figure 3), with a P-value of 0.003.
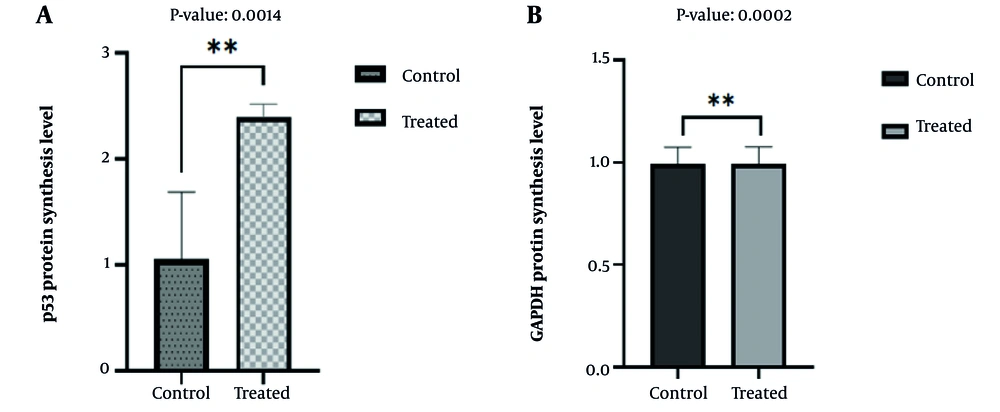
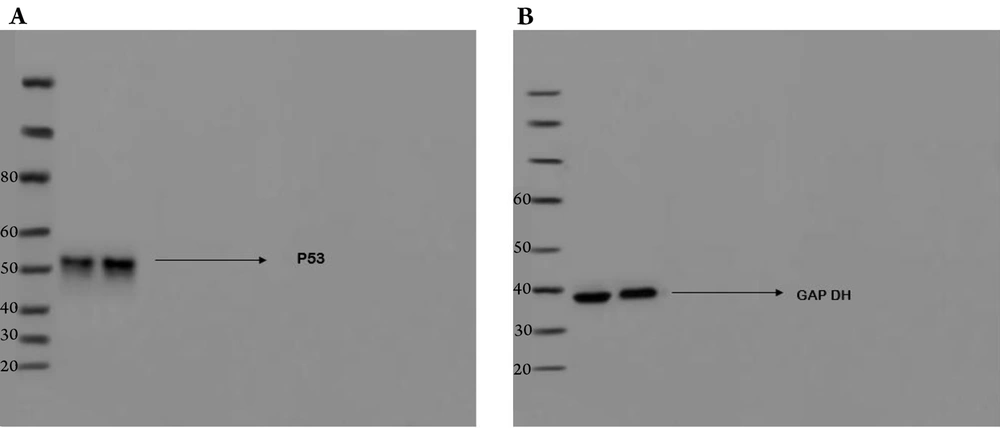
4.3. Effect of Nano-Curcumin on Cell Cycle Arrest: Western Blot Analysis of Increased p53 Expression
Analysis of p53 protein synthesis and expression by Western blot confirmed the flow cytometry results. The Western blot results showed that nano-curcumin increased p53 expression, which could induce apoptosis and cell cycle arrest in cancer cells (Figures 4 and 5).
5. Discussion
According to the World Cancer Statistics in 2020, with approximately 2.2 million new cases and about 1.8 million fatalities annually, lung cancer is the primary cause of cancer-related deaths in 185 countries for 36 cancers globally (4). Despite recent advances in surgery and chemotherapy, the prognosis remains extremely poor, with an overall survival rate of only 15%. TP53 abnormalities play a significant role in lung epithelial cell carcinogenesis, with TP53 gene mutations occurring in approximately 50% of non-small cell lung cancers (NSCLCs). Polyphenols not only regulate the expression of the p53 gene but also modulate p53 post-translational modifications, such as methylation, phosphorylation, acetylation, and ubiquitination, which collectively impact the function of p53 in response to DNA damage, apoptosis regulation, and cell cycle adjustment (25).
Polyphenols have been identified as significant components of natural products due to their unique chemical structure. Curcumin has been utilized in various cellular and protein research as a model polyphenolic compound, providing compelling evidence of its beneficial effects on health. Curcumin polyphenolic compounds are among the most promising substances for controlling cancer progression by managing numerous biological functions, including the cell cycle, cell death, proliferation, and differentiation (26). However, due to curcumin’s poor stability and absorption, its therapeutic use has been limited (27). To address these limitations, curcumin has undergone chemical modifications and new drug delivery methods, such as the creation of new compounds and nanocarriers, including liposomes, solid dispersions, micelles, and nanotechnology.
This research aimed to determine the effect of varying concentrations of nano-curcumin on the survival of A549 cells compared with the control group over 48 hours. According to the MTT assay, data showed that with increasing concentrations of nano-curcumin, the percentage of cell viability decreased in a dose-dependent manner. When lethal concentrations (IC50 concentration and higher) were reached, the decrease in cell viability was further pronounced. We demonstrated that curcumin can efficiently inhibit the spread of A549 cells by affecting various molecular processes, such as halting A549 cells in the G1/S phase and inducing apoptosis. Curcumin has a broad range of effects, which may contribute to its high anticancer efficacy. Therefore, curcumin is a potent suppressor of A549 cell survival and could be a novel therapeutic candidate for lung cancer treatment by affecting p53 expression and cell cycle control.
Our findings confirm the results of the study by Dash and Mukherjee, in which nano-curcumin induced further cell death (27). The reason for such observations requires further investigation, including the effect of nano-curcumin treatment on downstream molecular pathways, which is a limitation of this study. Future studies targeting the effects of chemotherapy dose reduction will further strengthen the findings of this investigation.
5.1. Conclusions
Although the impact of curcumin-loaded nanoparticles with different carriers, such as nanoliposomes and micelles, has been investigated in various studies, there is no report on the effect of nano-curcumin without any carrier directly on lung cancer cell lines. In this investigation, for the first time, nano-curcumin without any carrier or transporter was used. Analysis of the results shows an increase in p53 expression in the A549 cell line treated with nano-curcumin compared to the control, which was confirmed by the results of MTT, flow cytometry, and Western blot tests. It appears that nano-curcumin, by increasing the expression of genes like p53 that play a tumor suppressor role, prevents cell division, expansion, and migration of lung cancer cells by arresting the cell cycle. This process of increased expression occurs in the direction of apoptosis. Therefore, nano-curcumin may have an effective role in the treatment and improvement of lung cancer.