1. Background
Inflammatory markers and cytokines in patients with COVID-19 who experience severe illness, compared to those with mild cases, can alert physicians to include these factors in risk stratification models. Approximately 46% to 65% of patients require hospitalization in the intensive care unit (ICU), and 45% to 65% of those admitted to the ICU rapidly deteriorate and die due to respiratory failure. A decrease in glutathione (GSH) levels is associated with common aging features, pathological conditions, and smoking habits, which are significant risk factors for COVID-19 (1). Higher levels of GSH are linked to better resistance to viral infections and a more favorable response to such infections, with its deficiency playing a detrimental role in COVID-19 (2). The lack of GSH leads to the activation of the Von Willebrand factor (3) and the accumulation of reactive oxygen species (ROS), affecting clotting and platelet activation, disrupting endothelial function, and increasing the risk of thrombosis, particularly overcoagulation, a life-threatening effect in COVID-19 patients (4). Additionally, these patients experience immune dysfunction, granulocyte/monocyte abnormalities, blood vessel damage, and multi-organ dysfunction, potentially leading to death.
A study in Wuhan observed increases in neutrophils (83%), decreases in lymphocytes (35%), increased interleukin-6 (IL-6) (52%), and increased C-reactive protein (CRP) (84%) in patients (5). Oxidative stress is a key factor in tissue damage in severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection. High-sensitivity CRP (hs-CRP) is an inflammatory cytokine associated with tissue damage and infection response (6). CRP levels are often used to diagnose early pneumonia. Interleukin-6 is a recognized inflammatory marker consistently associated with COVID-19 progression (7). Mandel et al. demonstrated that IL-6 levels predict 30-day mortality in hospitalized COVID-19 patients with high sensitivity at specific thresholds (8). Ruan et al. also linked increased IL-6 levels to poor outcomes in severe COVID-19 cases (9). Additionally, IL-6 increases with viral load, making it a potential target for medical intervention in severe inflammatory cases.
Interleukin-10 (IL-10), another cytokine, helps regulate inflammation by decreasing pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) (10). Measuring IL-10 can assess inflammatory status, as it prevents overactive immune responses and aids recovery after infection. In COVID-19 patients, IL-10 increases before IL-6, making it an important early diagnostic marker (11). McElvaney et al. developed the Dublin-Boston score, based on IL-6 ratios, to predict clinical outcomes, demonstrating its use in identifying high-risk patients needing critical care or mechanical ventilation (12).
N-acetylcysteine (NAC), a precursor to glutathione, has been utilized in respiratory infections due to its antioxidant properties (13). Some studies suggest that allicin, a compound found in garlic, improves COVID-19 symptoms after two weeks of treatment (7). In another study, elderly patients receiving oral NAC for six months experienced fewer flu-like symptoms and shorter illness durations (14). Research indicates an inverse relationship between 25-hydroxyvitamin D and IL-6 and CRP levels. Vitamin D3 deficiency may increase the severity of COVID-19 and is also linked to the flu, chronic obstructive pulmonary disease, and upper respiratory infections (15).
The selection of hospitalized individuals in this research was motivated by several significant considerations. Hospitalized individuals face complex medical conditions that impact their treatment processes. The nature of these complexities creates both challenges and opportunities for expanding patient knowledge. The decisions patients make during hospitalization largely determine their health outcomes, making it essential for them to understand their care procedures. Additionally, hospitals provide an ideal setting to study and develop interventions aimed at improving patient knowledge. Enhancing patient education and empowerment are crucial components of patient-centered care in hospitalized settings. By conducting this research in hospitals, the authors recognize the advantage of accessing accurate medical records and the ability to assess the real-time effects of interventions on patient knowledge levels. These factors collectively justify focusing on hospitalized patients in this study, while also acknowledging the potential benefits that a broader population might bring to future research.
2. Objectives
Since COVID-19 continues to spread globally, and vaccines may not cover all variants, early diagnosis is essential for effective treatment and reducing mortality. This study focuses on evaluating glutathione redox status and inflammatory markers in ICU patients with SARS-CoV-2 infection to predict disease severity.
3. Methods
3.1. The Community and Statistical Sample
After obtaining permission from the research ethics committee of the University of Mohaghegh Ardabili (approval code: IR.UMA.REC.1400.049) and written informed consent from participants, a semi-experimental study was conducted. The study population included all patients with COVID-19 admitted to the ICU. The research sample size was calculated to include 16 individuals per group (10 men and 6 women) using G*Power software version 3.1.2, with an independent t-test, a statistical power of 0.97, an effect size of 0.7, and an alpha level of 0.005. All patients hospitalized in the ICU had at least 50% respiratory involvement. The age range for both groups was between 53 and 71 years. An inclusion criterion was a minimum of 3 days of hospitalization in the ICU, while an exclusion criterion was hospitalization for less than 5 days.
3.2. Measuring Technique
After collecting blood samples, serum was separated using a centrifuge at 3000 rpm for 10 minutes. The obtained serum samples were stored in a freezer at -80°C until testing. Samples were removed from the freezer and allowed to thaw at room temperature before determining the research markers according to the corresponding kit instructions.
To measure CRP, the DY1707 DuoSet enzyme-linked immunosorbent assay (ELISA) development kit from R&D Systems Inc. was used. Allicin levels were measured using the cysteine assay kit (catalog number MAK255) from Sigma-Aldrich. Interleukin-6 was measured using the DuoSet ELISA development kit (catalog number DY206-05) from R&D systems Inc. Interleukin-10 was measured using the DuoSet ELISA development kit (catalog number DY217B) from R&D systems Inc. The intra-assay variation coefficient and sensitivity for IL-6 were 4% and 0.094 pg/mL, respectively, and for IL-10, they were 6.2% and 19 pg/mL. Glutathione levels were measured using the glutathione assay kit (catalog number CS0260) from Sigma-Aldrich Inc. Vitamin D in the serum was measured using the Euroimmun kit from the United States via the ELISA method. Vitamin D deficiency was defined as serum levels between 10 and 20 ng/mL, based on established guidelines (16). All kits and materials were also allowed to reach room temperature for 2 hours before use.
3.3. Data Analysis Method
The analysis of normality was conducted using the Shapiro-Wilk test. To compare differences between groups, the independent t-test was employed. Additionally, Cohen's d effect sizes were calculated to ascertain the effect size of the independent test. The significance level for all statistical operations was determined using SPSS version 27. Significance was established at the level of the charts, which were also plotted using GraphPad Prism 9 software.
4. Results
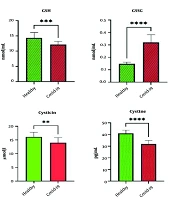
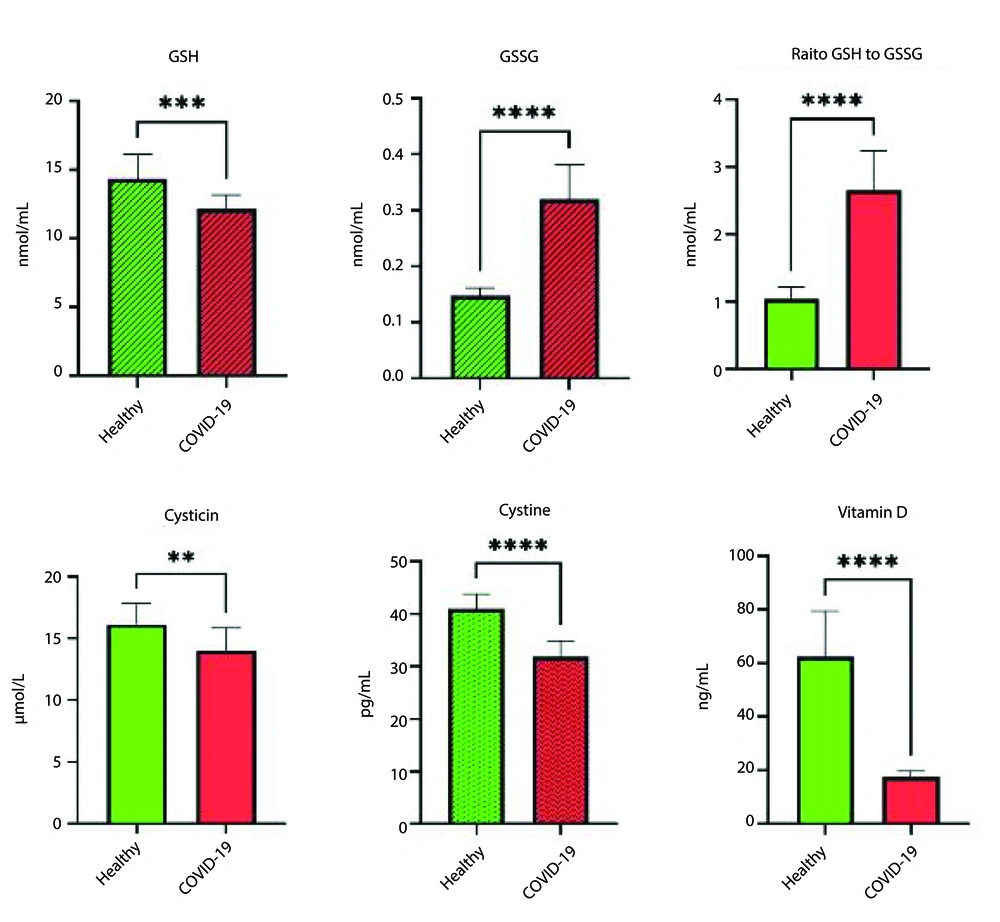
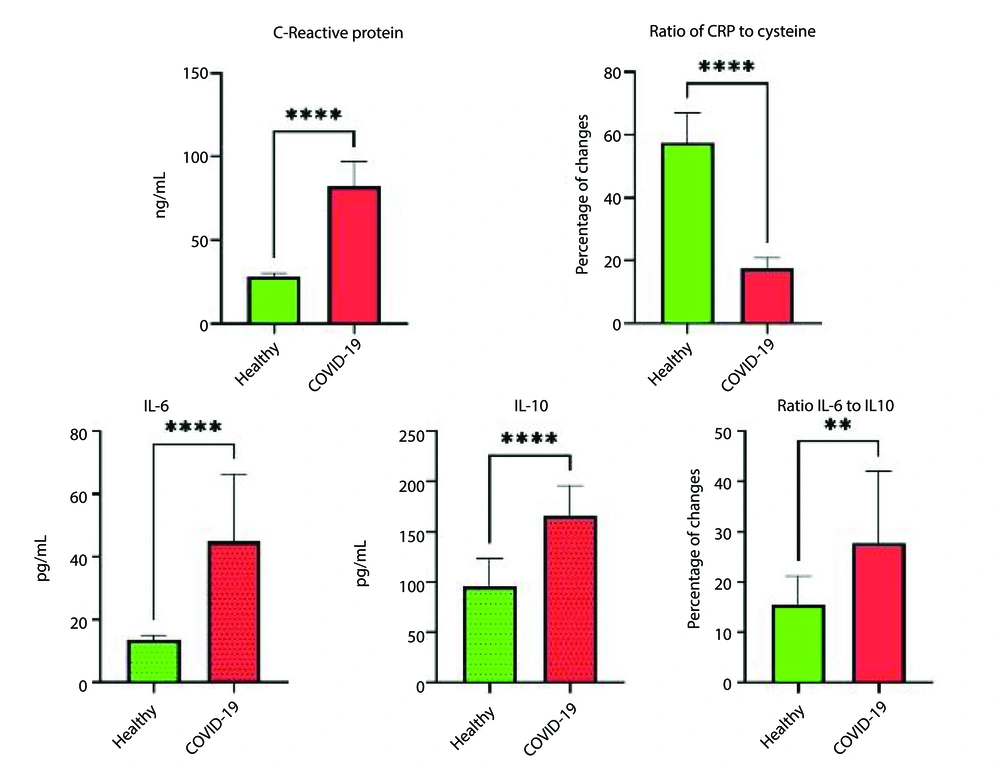
The results indicated that all patients hospitalized in the ICU exhibited over 50% respiratory involvement. The levels of reduced glutathione (GSH) in patients with COVID-19 were significantly lower, while oxidized glutathione (GSSG) levels were higher compared to healthy individuals (P ≤ 0.05). Additionally, the study results demonstrated that CRP levels in COVID-19 patients were significantly higher than those in healthy individuals (P ≤ 0.001). Moreover, allicin levels in COVID-19 patients were significantly reduced (P ≤ 0.004). Furthermore, the t-test results revealed a significant difference in cysteine levels between hospitalized COVID-19 patients and healthy individuals (P ≤ 0.004). The levels of IL-6 and IL-10 in ICU patients were significantly increased compared to healthy individuals (P ≤ 0.05). Additionally, all ICU patients exhibited vitamin D deficiency, with levels ranging from 10 to 20 ng/mL, whereas healthy individuals had levels above 66 ng/mL (Table 1).
| Indicator | Healthy | COVID-19 | Effect Size (95% CI) | t | F |
|---|---|---|---|---|---|
| GSH (nmol/mL) | 14.35 ± 1.78 | 12.14 ± 1.02 | 1.45 (0.66 to 2.36) | 4.03 | 0.001 |
| GSSG (nmol/mL) | 0.14 ± 0.01 | 0.32 ± 0.06 | 0.05 (-5.13 to -2.57) | -10.22 | 0.001 |
| CRP (mg/L) | 28.2 ± 19.02 | 84.13 ± 16.91 | 9.75 (-7.47 to -3.97) | -14.32 | 0.001 |
| IL-6 (pg/mL) | 13.54 ± 1.56 | 45.96 ± 21.91 | 15.53 (-3.04 to -1.10) | -5.32 | 0.001 |
| IL-10 (pg/mL) | 90.02 ± 23.63 | 162.11 ± 32.56 | 28.45 (-3.65 to -1.47) | -6.46 | 0.001 |
| L-cysteine (µM) | 16.14 ± 1.70 | 14.00 ± 1.87 | 1.77 (0.38 to -1.47) | 3.19 | 0.004 |
| Cystine (pg/mL) | 13.1 ± 47.46 | 43.21 ± 19.56 | 2.86 (2.03 to 4.31) | 8.43 | 0.001 |
| Vitamin D | 66.16 ± 43.90 | 17.2 ± 43.20 | 12.05 (2.72 to 5.37) | 10.75 | 0.001 |
Comparing the Glutathione Redox and Some Inflammatory Indicators in Patients with COVID-19 and Healthy Controls a
The results of this study also indicated that, in addition to the levels of GSH and GSSG, there is a significant difference in the GSH/GSSG ratio between COVID-19 patients and healthy individuals (1). Moreover, the independent t-test revealed that IL-6 levels had doubled, and IL-10 levels had increased by one-and-a-half times in COVID-19 patients. Additionally, the comparison of IL-6 to IL-10 ratios showed that this ratio is significantly higher in COVID-19 patients than in healthy individuals. Furthermore, the ratio of CRP to cysteine demonstrated a significant difference between COVID-19 patients and healthy controls (P ≤ 0.001) (Figures 1 and 2).
5. Discussion
This study investigated the glutathione redox status and inflammatory markers in ICU patients with acute respiratory syndrome due to COVID-19. The results showed that GSH levels were significantly lower and GSSG levels significantly higher in COVID-19 patients compared to healthy individuals. Previous studies have indicated that viral replication, particularly in the Coronaviridae family, triggers the unfolded protein response (UPR) and endoplasmic reticulum (ER) stress, leading to the activation of apoptosis in host cells (17, 18). These cellular responses, along with processes like autophagy and mitophagy, are regulated by cellular redox mechanisms (19). The mechanisms behind GSH depletion in infected cells remain unclear. Possible factors include the release of a tripeptide during viral exocytosis and the binding of cellular cysteine with viral proteins, which interferes with GSH biosynthesis. This disruption affects the redox balance of proteins (20). Any residual cysteine may maintain protein homeostasis through glutathionylation, but this can cause protein damage, ER stress, and dysregulated signaling, leading to cell death (21). These observations suggest that SARS-CoV-2 infection alters GSH metabolism, disrupting redox balance and affecting extracellular thiols (22). Further research into these processes may uncover new therapeutic strategies for COVID-19 treatment.
The study also found that CRP levels were significantly higher in COVID-19 patients compared to healthy individuals. The CRP plays a role in enhancing phagocytosis, helping to clear pathogens from the body. Elevated CRP levels have been linked to severe pneumonia, making it a useful marker for early diagnosis of respiratory infections. Severe COVID-19 cases had higher CRP levels than non-severe cases, and non-survivors had higher CRP levels than survivors (23). Matsumoto et al., in line with this study, demonstrated the value of CRP levels in severe pneumonia (24). This underscores CRP's value in distinguishing severe from non-severe cases and in predicting mortality. High CRP levels were closely associated with ICU admission and death in COVID-19 patients, making it a critical early indicator of respiratory damage and disease severity (24). Increasing CRP levels are a risk factor for ICU hospitalization and death in COVID-19 patients, reflecting respiratory lesions and disease severity in the early stages of the disease.
The current study also showed that IL-6 levels were significantly elevated in ICU patients with COVID-19. Another study supported this finding in their meta-analysis, showing that IL-6 levels increase in severe cases and are associated with adverse outcomes (12). The progression from initial SARS-CoV-2 infection to severe disease could result from excessive immune responses and autoimmune damage. This highlights the need for clinical trials to explore the role of immune modulation, particularly through IL-6 inhibition, in treating severe COVID-19 cases.
Similarly, IL-10 levels were significantly higher in ICU patients. Park and Skerrett found that IL-10 concentrations were higher in ICU patients than in non-ICU patients, suggesting a potential anti-inflammatory role of IL-10 in suppressing excessive immune responses (10). This is thought to be a negative feedback mechanism for suppressing inflammation. We propose that using a neutralizing antibody to inhibit IL-10 and limit its potential effects on the immune system in the early stages of COVID-19 may be valuable for testing. Interleukin-10 may also promote inflammation induced by viral sepsis observed in some patients with severe COVID-19 (7). However, IL-10 may contribute to viral sepsis and disease severity. Its role in activating cytotoxic CD8+ T-cells could exacerbate immune hyperactivation, worsening the disease. Although speculative, IL-10's dual role in promoting inflammatory cytokine production and T-cell activation could lead to deadly immune pathology in COVID-19 patients.
The study further noted a significant increase in the IL-6 to IL-10 ratio in COVID-19 patients, rising from 36% in healthy individuals to over 64% in ICU patients. A comprehensive analysis of cytokine biomarkers, such as IL-6, TNF-α, and IL-10, or their ratios, could provide valuable insights into the inflammatory state of COVID-19 patients. Current studies suggest that measuring pro-inflammatory cytokines as biomarkers may help manage COVID-19 by assessing risk, monitoring disease progression, determining prognosis, and predicting treatment responses. Interleukin-6 (IL-6) and IL-10 levels, particularly their ratio, were found to accurately predict disease severity (11). Given the pathologic role of prolonged IL-6 elevation in cytokine release syndrome (CRS) and the associated mortality, clinical trials are exploring the potential of IL-6/IL-6R inhibitors as treatments for COVID-19. On the other hand, high IL-10 levels may serve as an anti-inflammatory mechanism, potentially acting as a negative feedback loop to counterbalance the pro-inflammatory cytokine surge (11).
The study also revealed that allicin levels were significantly reduced in COVID-19 patients. Allicin, a thiol-containing amino acid and glutathione precursor, has strong antioxidant properties that may be beneficial in various pathological conditions. N-acetylcysteine, a well-known GSH precursor, has shown positive effects in treating viral respiratory infections by inhibiting the expression of IL-8, IL-6, and TNF-α in alveolar cells infected with respiratory viruses (12). Some research with multivariate regression analysis showed that severe patients receiving standard care treatment (without NAC) were associated with increased mortality (25). In the control group with an average age of 64 years, mortality above 30% was registered for 28 days. The mortality rate reported for hospitalized patients of a similar age and those with previously reported COVID-19 pneumonia were alike (26).
The potential mechanisms for the beneficial effects of NAC have been investigated in several in vivo/in vitro studies. Beyond its well-established action, various additional mechanisms have been proposed for its antioxidant and anti-inflammatory effects as a glutathione precursor: (1) NAC decreases the mRNA expression of the nucleotide-binding domain and leucine-rich repeat protein 3 (NLRP3) inflammasome, reducing pro-inflammatory cytokine expression and release from active mononuclear phagocytes (27); (2) inhibits the release of endotoxin-induced IL-1β, IL-8, and TNF-α (28); (3) improves intestinal barrier dysfunction, preventing systemic endotoxemia and the inflammatory response, while prior studies showed that COVID-19 is related to intestinal barrier dysfunction and systemic endotoxemia (1); (4) reduces programmed cell death with an inhibitory receptor death protein 1, increasing the expression and lifespan of CD4+ and CD8+ lymphocytes (29). Moreover, NAC may exert a direct antiviral impact against SARS-CoV-2. Previously, it was shown that early NAC cut-off in COVID-19 was associated with the occurrence of experimental inflammation indicators (26).
Vitamin D levels were also significantly lower in ICU patients. Seifi-Skishahr and Nabilpour found an inverse relationship between Vitamin D levels and the incidence of severe COVID-19 (7). Vitamin D plays a critical role in stimulating antimicrobial peptides in the immune system, providing protection against bacterial, viral, and fungal infections (1). It also moderates adaptive immune responses. A study reported that Vitamin D supplementation can reduce the number of respiratory infections by two-thirds in affected individuals (30). Since this study was conducted in winter, reduced sun exposure likely contributed to Vitamin D deficiency. This suggests that Vitamin D supplementation should be prioritized during autumn and winter to help reduce COVID-19 complications.
This study has several limitations. First, the sample size was small, including only 16 COVID-19 patients and a healthy control group. Second, the cross-sectional design limits causal inference. Third, insufficient control of variables affecting the outcomes may have influenced the results. Additionally, this study did not examine gender differences. Future studies should focus on gender-based analysis to gain a more detailed understanding of immune responses and COVID-19 outcomes. Furthermore, the lack of long-term follow-up and the limited assessment of immune markers may impact the validity and generalizability of the findings.
5.1. Conclusions
The measurement of glutathione redox status, as well as inflammatory markers like IL-6, IL-10, and CRP, can serve as crucial predictors for assessing the severity and progression of COVID-19 in ICU patients. The study also indicates that NAC supplementation could help alleviate inflammation and oxidative stress, potentially improving patient outcomes. Furthermore, the study highlights the importance of Vitamin D levels in relation to COVID-19 severity. However, the small sample size and cross-sectional design of the study limit the conclusions, indicating the need for further research to validate these results and explore their clinical application.