1. Background
Nitrate contamination due to its high solubility is largely dissolved in the surface and ground waters (1, 2). The sources of nitrate (point and nonpoint sources) include agricultural runoff (3), untreated disposal of sanitary and industrial wastes, leakage from septic tanks, landfill leachate, use of animal manure, and wastes from air pollution control devices to remove NOx (4). Contaminated effluent without any treatment into the environment are considered as an environmental threat (5, 6).
Ground water is widely used for drinking. Continual uptake of nitrate by drinking water can lead to problems such as blue baby syndrome in infants (2, 7-9).
The recommended standards of nitrate (100 mg NO3−/L) in drinking water has been suggested by the European community (1) and (10 mg NO3-/L) by EPA (10). So, it is necessary to treat carefully and efficiently the water sources and wastewater (11, 12).
Methods of contamination removal from natural waters and industrial waste waters has gained a lot of attention that leads to the production of high-quality water (13). Elimination of nitrate from water can be done by ion exchange, reverse osmosis, biological and chemical reductions, and adsorption (4, 14). The use of traditional treatment techniques are very expensive (15), continuous use of chemicals may cause further environmental damages (16). Hence, the search for a low cost and readily available adsorbent has led to the use of agricultural by-products and or biological sources as adsorbents (16, 17).
Recently, agricultural by-products, e.g. peat and pith, waste cellulose, apple pomace, and wheat straw, are widely used and studied for removal of dyes (18), heavy metals (16), and so on from wastewater.
In this study, rice chaff was used because of its abundant floristic fiber, protein, and some functional groups, e.g. carboxyl, hydroxyl, amidogen, which make possible the biosorption processes (18). The adsorbent chaff is obtained from agricultural by-product, so they can be used extensively (1).
Han et al. (18) used the chaff for removal of methylene blue (MB) from aqueous solution and found that a total of 95% of MB was removed within 60 minutes after the start of experiment with the initial MB concentration of 30 mg/L and the temperature of 298 K (24.85°C). The pH had no effect on MB removal as pH increased from 4 to 11.
Rezaei Kahkha et al. (19) used citrullus colocynthis powdered fruits for cadmium removal from an aqueous solution. They indicated that the best condition for cadmium removal is obtained at pH 4.5, initial cadmium concentration of 31.22 g/L, and adsorbent dosage of 3.75 g/L.
Jorgetto et al. (20) studied the ability of root husks powder for the removal of Cu (II) from natural river water and found the most favorable adsorption pH at a range of 3 - 6 and the most adsorption capacity at 0.14 mmol/g.
Ghaneian et al. (21) found that by using the pomegranate seed powder for removal of reactive red 198 dye, the percentage of adsorption decreased with the increase in adsorbent dose and contact time and the increase in initial dye concentration (from 25 to 50 mg/L); moreover, the results indicated that pseudo-second-kinetic model (R2 > 0.99) has a good agreement with data.
Fadaei et al. (22) used the Jujube fruit for removing chromium, indicating that the maximum Cr removal was achieved on pH < 2.
Shamohamadi (23) showed that by using chaff and activated carbon for removing the cadmium, the initial concentration and equilibrium time for both absorbent decreased. In this study, the equilibrium time for activated carbon (AC) and chaff lasted 45 and 60 minutes. The maximum equilibrium time for AC and chaff continues up to 120 and 90 minutes, respectively.
2. Objectives
The aim of this study was to measure the potential ability of rice chaff for the removal of nitrate ion from water in different pH levels, absorbent dosages, sorption times, and temperatures.
3. Materials and Methods
3.1. Preparation of Absorbent
Fresh chaff was collected from farms. Then, it was screened to separate geometrical sizes of 0.45 - 0.6 mm with an average particle size of 0.5 mm. It was washed for a few minutes with distilled water and then with detergent. Next, it was dried for 3 hours at 90°C in the oven.
3.2. Preparation of Nitrate Solution
The chemical used for the study was pure nitrate (200 mg/L) that was prepared with standard instruction of water laboratory.
3.3. Method
This is a cross-sectional study conducted in laboratory scale. All of the materials were supplied by Merck Company in Germany. The data obtained in this study was used to calculate the percentage of nitrate uptake quantity using the following expression:

Where c0 = the initial nitrate concentration (mg/L) and ce = the residual nitrate concentration (mg/L).
The number of sample was found 36 with full factorial method. Biosorption tests were conducted at various contacting times (5 and 30 minutes) at the primary concentration of nitrate of 200 mg/L and the amount of chaff was 1 and 3 g. Temperature and pH were changed at 300°C, 400°C, and 500°C; and values of 3, 7, and 10, respectively (Table 1).
| Initial Concentration of Nitrate, mg/L | Amount of Chaff, g | Contact Time, min | Temperature, °C | Solution pH |
|---|---|---|---|---|
| 200 | 1 | 5 | 300 | 3 |
| - | 3 | 30 | 400 | 7 |
| - | - | - | 500 | 10 |
Parameters Studied in the Removal of Nitrate by Chaff
Admixture was performed at a constant speed of 180 rpm. The adsorbate and the adsorbent were separated by using filter paper. The concentrations of residual nitrate were measured in the remaining solution and analyzed by using a UV spectrophotometer at a wavelength of maximum absorbance (220 nm) and then the percentage of absorbance was calculated. The experiments were conducted in two series; in part one, we used 18 erlen.
3.4. Effect of Chaff Dose and Contact Time on Biosorption
Experiments were carried out by contacting 1 and 3 g of chaff particles with initial concentration of nitrate 200 mg/L. Admixture contact times were 5 and 30 minutes at a constant agitation speed of 180 rpm.
3.5. Effect of pH on Biosorption
The effect of pH on the amount of nitrate removal was analyzed at the pH values of 3, 7, and 10. The pH was controlled using 0.1 mol/L HCl solution and NaOH. The pH was measured with pH meter (pH lab-Metrohm, Swiss 827).
3.6. Effect of Temperature on Biosorption
The effect of temperature on the amount of removed nitrate was measured at 300°C, 400°C, and 500°C temperatures.
4. Results
Biosorption of nitrate depends on pH solution, amount of chaff, temperature and contact time. The results of biosorption and removal efficiency at different shaking times, pH, and temperature are shown in Figures 1 - 4.
The effect of pH on removal efficiency was studied by changing the pH level from 3 to 10 and amount of chaff (1 and 3 g), temperature (300°C, 400°C, 500°C) and contact time (5 and 30 minutes).
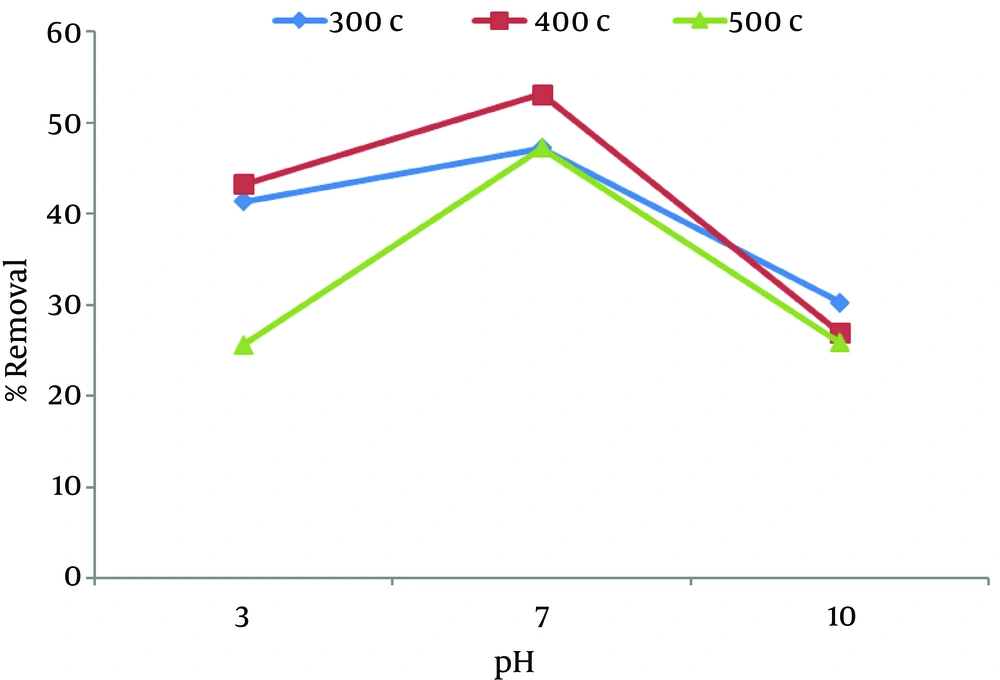
Figure 1 shows the removal of nitrate at a constant nitrate concentration of 200 mg/L, adsorbent concentration of 1 g, and contact time of 5 minutes. It showed that, the removal efficiency increased from pH = 3 - 7 and then decreased with the increase in pH in all temperatures. The maximum adsorption rate of nitrate (53.1%) was seen at pH = 7 at 400°C.
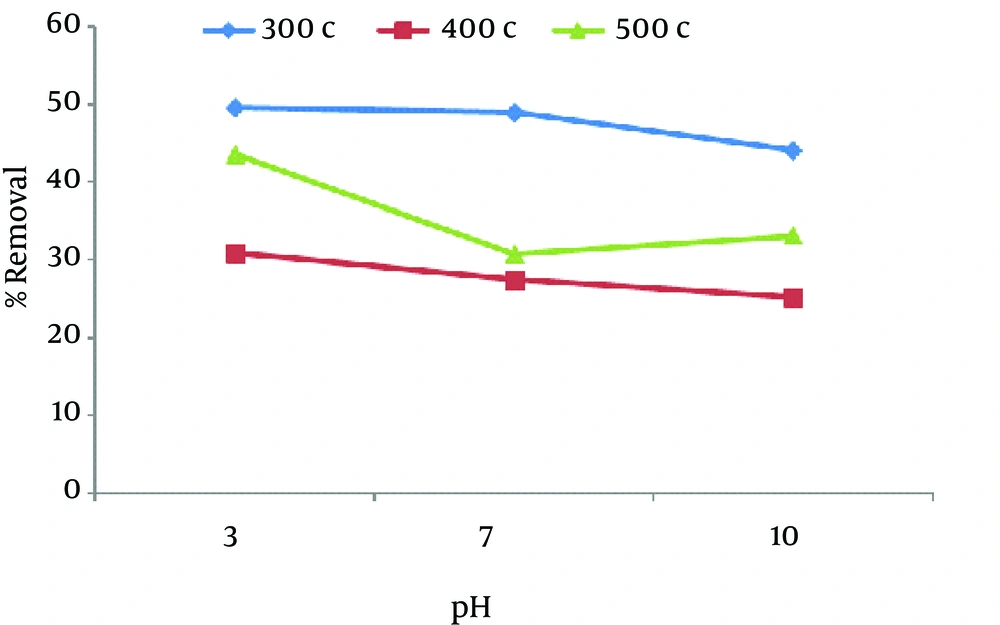
Figure 2 shows the adsorption of nitrate using 1 g of chaff in 30 minutes. As shown in Figure 2, when the pH of the solution changed from 3 to 10, the removal percentage decreased.
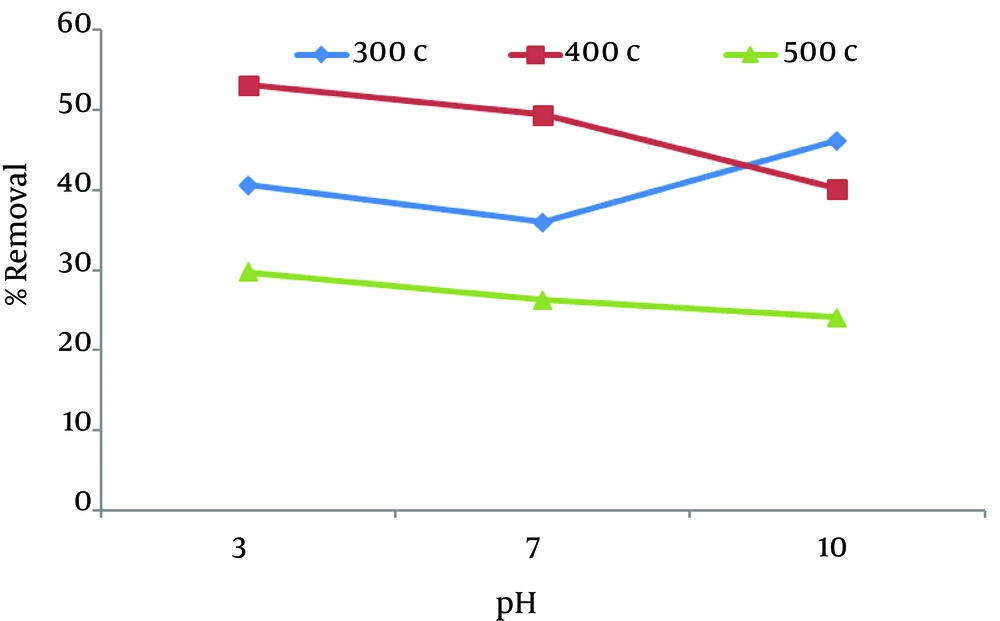
As shown in Figure 3, the maximum adsorption of nitrate (53.2%) was seen at pH 3, temperature of 400°C, at a constant nitrate concentration of 200 mg/L, adsorbent concentration of 1 g, and contact time of 5 minutes. The finding showed that the removal rate decreases by increasing pH, except at 300°C.
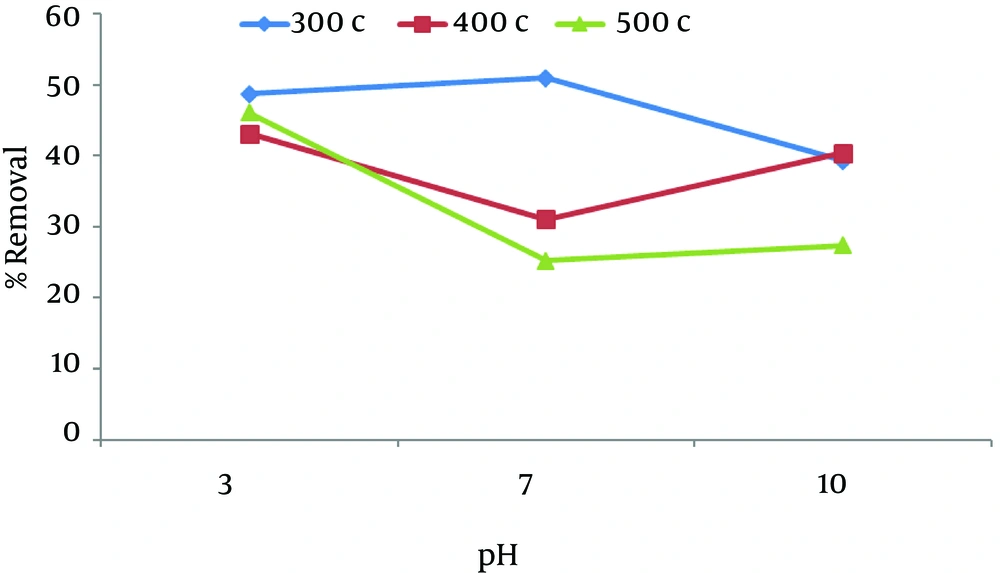
According to Figure 4, the lowest removal rate is in pH 7 at 400°C and 500°C and the removal rate increases on both sides with a mild angle, but opposite behavior was observed at 300°C. The maximum removal rate occurred in pH 7 at 300°C and the removal efficiency decreases with a mild angle.
As shown in the figure pH of the solutions has been identified as one of the most important parameters, that governs adsorption in different temperatures. The highest adsorptive capacity of nitrate (53.2%) was observed in pH 3 by using 3 g of chaff at 400°C and with 5 minutes of contact time.
5. Discussion
Adsorption technology is used successfully in removing various types of inorganic anions such as fluoride, nitrate, bromate, and perchlorate from water via applying different substance as adsorbents. Adsorption process is much better in water treatment because of its convenience, simplicity of operation, and simple design, although biological denitrification and IX (Ion exchange) is proposed by The World Health Organization (WHO) as nitrate removal methods and IX, RO (reverse osmosis), and ED (electro-dialysis) have been approved by US EPA as Best Available Technologies (BAT) (2). BATs are to some extent expensive, so in this study the adsorption process was performed using chaff.
5.1. Effect of pH Solution on Biosorption
The value of pH is one of the most important environmental factors that influences adsorption process and anion adsorption (17). On the other hand, pH strongly influences the biosorption availability of the nitrate (16).
To determine the optimum pH for the maximum removal of nitrate, the experiments were preformed over a pH range of 3 to 10. As Figures 1 - 4 shows, in majority of samples when pH decreases from 10 to 3, the removal efficiency increases because chaff surface takes positive charges and as a result adsorption increases. In general, This may be due to the competition between active sites by OH− ions for nitrate adsorption and the electrostatic repulsion of anionic nitrate by the negatively charged chaff surface at higher pH (2, 8). In fact, when pH is high, the whole chaff surface becomes negative (16) and adsorption decreases. Different studies have shown that efficiency increases in acidic solutions and pH is an important controlling parameter.
Jorgetto et al. (20) has shown that by using husks root powder for the removal of Cu (II) from natural river water, optimum adsorption pH is in the range of 3 - 6 and the amount of adsorption in this range is about 0.08 mmol/g. Afkhami et al. (24) studied the effects of functional groups on the adsorption of NO3− and NO2− by carbon cloth at nearly neutral (pH ~ 7) solutions. The results have shown that the treatment of carbon cloth with acid produced positive sites on the carbon cloth and caused an increase in electrostatic adsorption of anions. Nemr (25) showed that by using the husk carbon for removal of Cr (VI) from wastewater, maximum chromium uptake (about 94%) was obtained at pH 1.0.
In another study, aiming at removing the Reactofix Golden yellow 3 RFN from aqueous solution using wheat husk, Gupta et al. (26) revealed that maximum uptake of dye occurs at pH 2 (26). Moreover, Bansal et al. (27) showed that for the removal of Cr (VI) using agricultural waste, maximum metal removal was observed at pH 2.0. The efficiency of boiled and formaldehyde treated rice husk for Cr (VI) removal were 71.0% and 76.5%. All of these studies showed the same results that efficiency increases in acidic solutions.
5.2. Effect of Contact Time and Temperature on Biosorption
One parameter that affects adsorption is temperature. In this study, 3 different temperatures were used. Temperature has a positive but limited effect on the removal efficiency. Very low or high temperature has adverse results on the efficiency rate (5). In other words, the removal rate reduced below and above the optimum temperature (6). Figure 3 shows the highest removal efficiency rate (53.2%) at 400°C.
In the same study, Han et al. used chaff to remove methylene blue from aqueous solution, showing that when temperature changed from 298 K (24.8°C) to 333 K (60.6°C), removal of MB increases and the maximum adsorption (95%) was observed at 333 K (60.6°C) (18).
By using the rice husk for removal of 2,4-dichlorophenol, Akhtar et al. (28) reported that the maximum absorption (98 ± 1.2%) was achieved in 303 K (29.85°C) when temperature changed from 283 K (9.8°C) to 323 K (49.8°C). This research shows that by increasing the temperature up to a specific degree, adsorption increases and compared to this study, similar results were obtained. It is worth mentioning that optimum temperature in this study was lower.
To study the optimum time and its effect on nitrate removal, experiments were performed at 5 and 30 minutes. As shown in Figure 3, rapid adsorption occurred in the initial minutes. This figure shows that the maximum removal rate (53.2%) reached at 5 minute and then the rate of adsorption got slower. The fast adsorption in the first few minutes was probably due to the initial concentration among adsorbate in solution and the number of empty sites present on the adsorbent exterior at the beginning (17). Khaled et al. (29) reported that in removal of direct yellow 12 by orange peel carbon, more than 75% removal of dye concentration observed in the first 10 minutes.
5.3. Effect of the Amount of Chaff on Biosorption
The results demonstrated that change in the amount of initial chaff influenced the biosorption rate. As Figures 1 - 4 shows, by increasing the amount of adsorbent from 1 to 3 g while keeping the initial nitrate concentration constant (200 mg/L), the efficiency of nitrate removal increases. This can be explained by the fact that the higher adsorbent mass can provide more surface area and more adsorption sites, including functional groups (13). In fact, removal efficiency increased by increasing the adsorbent dosage because of the complex formation between adsorbent and adsorbate are optimum (19). This finding agrees with the results of previous studies.
In the same research by Han et al. (18) on removing methylene blue by chaff, it was indicated that as the dose of chaff increased, the percentage of MB sorption increased from 82.5% to 97.0% when the adsorbent load increased from 2 to 12 g/L.
Also, Akhtar et al. (28) indicated that the percentage of sorption increases rapidly by increasing the amount of the sorbent from 0.025 to 0.1 g and the maximum sorption (98 ± 1.2%) was achieved in 0.1 g. In another study on removal of Reactofix Golden yellow 3 RFN from aqueous solution using wheat husk, Gupta showed that by increasing the wheat husk from 10 g/L to 35 g/L, the removal efficiency increased (26).
Bansal et al. (27) have also shown that the removal of Cr (VI) by BRH (pre-boiled rice husk) ranged from 33.2% to 71% with various adsorbent doses of 4.0 g to 20.0 g/L and the removal of Cr (VI) by FRH (Formaldehyde treated rice husk) ranged from 38% to 76.5% with various doses of 4.0 g to 20.0 g/L. Somasekhara Reddy et al. (30) studied the removal of dyes by Bengal gram seed husk. It was shown that the uptake of dyes by the adsorbent rose when adsorbent mass increased from 0.1 to 0.2 g.
In this study, removal of nitrate from aqueous solution using rice chaff was investigated under different experimental conditions. The amount of nitrate adsorbed into chaff depends on pH of the solution, amount of chaff, adsorptive time, and temperature.
Adsorption into chaff indicates that acidification has a positive effect on the adsorption process. The percentage of removal increased when pH of the solution decreased from 10 to 3. Increase in temperature up to 400°C and increase in the amount of chaff can increase the removal efficiency. About 53.2% of the total nitrate (maximum adsorption) was removed within 5 minutes after the initial start of experiment when pH was 3, temperature was 400°C and amount of chaff was 3 g.
Therefore, among various materials that are low cost and readily available, rice chaff has good properties for adsorption. It has abundant floristic fiber, protein, and some functional groups, e.g. carboxyl, hydroxyl, amidogen, which can make adsorption processes possible (18). When biosorption occurs, ions-exchange or other mechanisms occur at the same time. In addition, adsorption/desorption recycles into chaff indicates that acidification has some advantages in the removal of nitrate and ion-exchanged mechanism participates in nitrate binding on chaff. As biosorption is a very complex process, further studies are needed in this regard.