1. Background
Multiple sclerosis (MS) is a chronic, immune-mediated and the most common inflammatory demyelinating disease of the central nervous system (1). Disease onset usually occurs in young adults, and more in females. It begins between the ages of 20 - 35 years old, but can also appear in children and old ages rarely. A wide variation exists in the prevalence of MS in different geographical regions, which ranges between 2 and 150 per 100,000 people (2, 3). The female to male ratio in young people is 3:1 and in people over fifty, MS affects both sexes almost equally (4). Multiple sclerosis has a complex etiology and still unknown causes. Researchers believe that MS is a multifactorial disorder, in which environmental factors are hypothesized to interact with genetically susceptible individuals (5). Kurtzke et al. (6) classified geographical areas for three regions by prevalence: (a) high > 30 per 100,000, (b) intermediate (5 - 25 per 100,000), and (c) low risk < 5 per 100,000. Based on such expectations, Middle-Eastern countries had been generally classified as low risk areas, but to date, several studies in this region report the higher risk (7). There are four subtypes of MS: relapsing-remitting (R.R), which implies 85% - 90% of individuals with MS; this subtype is more common in women than men. Secondary progressive (S.P), describes those with initial relapsing-remitting MS, who then begin to have a progressive neurologic decline between acute attacks (10 - 25 year after disease onset) (8). Primary progressive (P.P) subtype describes the approximately 10% - 15% of patients, who never have remission after their initial MS symptoms; this kind is more common in men than women and the age of onset is later than others (8, 9). Progressive-relapsing MS is the least common of all subtypes and from the onset, has a steady neurological decline and clear superimposed attacks (9). The prognosis of a person with MS depends upon the subtypes of the disease, sex, age, and initial symptoms and the degree of disability. Viral infections, far from the equator, vitamin D deficiency, severe stress, smoking, diet, pregnancy and hormonal disorders are main environmental risk factors; however, opposite results were seen in different studies (3, 10-12). In many reports, there is an increasing rate of MS between peoples with high socioeconomically status (3). The disease usually presents with sensorial and visual symptoms. Of course, in some cases the initial symptoms are different (13). Many researchers emphasize that kind of initial symptoms influences the course and disease prognosis; so that, optic neuritis and other sensory symptoms are associated with a better prognosis than those who have cerebellar or motor involvement in the beginning of the course. In Iran, from 1993 to now the prevalence of MS became 3 - 6 times (14). Multiple sclerosis poses a significant burden on health care costs and affects the productive capacity of work and quality of life years of those affected and increase the number of handicaps and disabled persons. Although the reasons why incidence of the disease is increasing are unknown, there are major implications for health care provision, because life time costs of MS exceed £1 million per case in the UK (1, 4). Some researchers believe that description of MS subtype in different regions can help to determine the environmental risk factors (15). Based on the division of Kurtzke, Iran lies within a low risk zone (less than 5 per 100000) of MS prevalence (10, 12). Although little is done about the epidemiology of MS in Iran, recent surveys in different parts of the Iran have demonstrated diverse results and somewhat, increased frequency of the disease from low to medium (16).
2. Objectives
The purpose of this study was to determine the prevalence, demographic features, clinical characteristics or subtypes of MS, initial symptoms at onset and the correlation between demographic characteristics and clinical course of MS in the north of Khuzestan.
3. Patients and Methods
This quantitative correlational study was conducted in seven northern cities of Khuzestan Province including: Dezful, Andimeshk, Shush, Shushtar, Masjed Soleiman, Lali and Gutevand, during April 2012 to August 2012. Partcipants were selected using the purposeful sampling. The target population consisted of all cases were recruited through neurology clinics, registration part of the MS Society of Ahvaz and Dezful, and neurology department of the general hospitals in northern cities of Khuzestan. Data were collected by completing a face to face questionnaire. Demographic data include age, sex, date of birth, marital and occupational status, number of siblings and children, home address, and smoking status. Moreover, clinical information, recorded by a trained general practitioner through an interview with the patient, provide the time of onset, diagnosis and presenting symptoms of the disease, pattern of progression, and clinical course. To prevent duplications of the data, a single ID number was dedicated to each patient.
3.1. Ethics
All of the patients signed a form confirming the consent for participation in the research and obtained data were kept as confidential. Moreover, in all stages of the study, the last version of the declaration of Helsinki was followed by the researcher, and the institutional ethics committee approved the use of the clinical information (Code 79/a, date 2012.14.4).
3.2. Statistical Analysis
Data were analyzed using SPSS version 17.0 (SPSS Production Facility, and Chicago, Illinois, USA). The chi-square test and independent t-test were used for data analysis.
4. Results
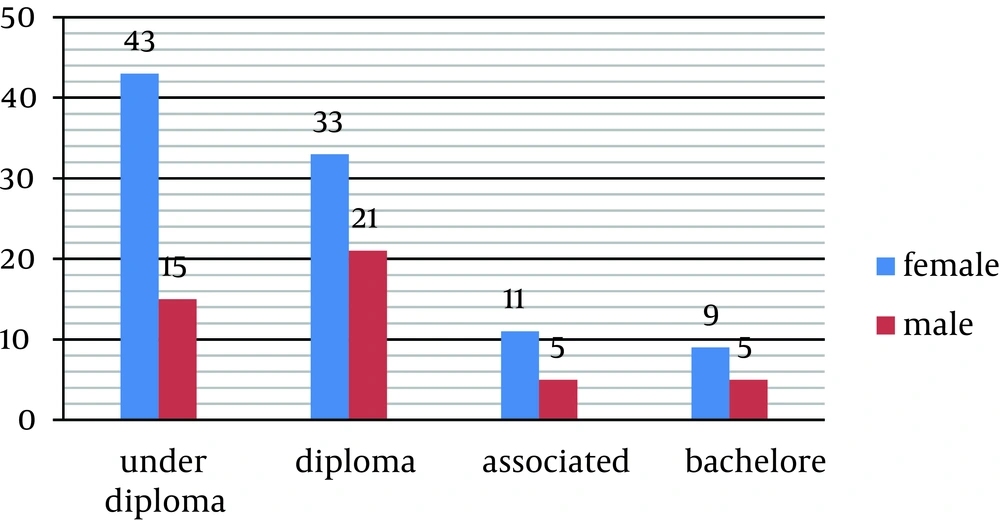
Overall, 142 patients with MS disorder were detected in the seven northern towns of Khuzestan Province in Iran (Dezful, Andimeshk, Shush, Shushtar, Masjed Soleiman, Lali and Gutevand). According to the data obtained from 2012 census, the estimated population of these towns was 957133; therefore, the prevalence of MS was 15 per 100,000. Biographical data of the patients have been shown in Table 1. Data showed that 62% and 38% of the cases were married and single, respectively. Furthermore, 78% had diploma degree and below this level of literacy and 81% were housekeeper or unemployed (Figure 1).
| Variations | Frequency |
|---|---|
| Gender | |
| Female | 96 (67.6) |
| Male | 46 (32.4) |
| Marital Status | |
| Single | 54 (38) |
| Married | 88 (62) |
| Occupation (female) | |
| Housewife | 64 (66.7) |
| Employed | 7 (7.3) |
| Unemployed | 24 (25) |
| Occupation (male) | |
| Employed | 6 (13) |
| Unemployed | 12 (26.1) |
| Immigration to Khuzestan | |
| No immigration | 100 (70.4) |
| > 16 y | 23 (16.2) |
| < 16 y | 19 (13.4) |
| Smoking Status | |
| Non-smoker | 132 (93) |
| Smoker | 10 (7) |
| Educational Status | |
| Under diploma | 58 (40.8) |
| Diploma | 54 (38) |
| Associated | 16 (11.3) |
| Bachelor | 14 (9.9) |
| Family Income | |
| ≥ 6000000 Rial | 92 (64) |
| 6000000-9000000 | 28 (19) |
| ≤ 9000000 | 24 (17) |
Demographic Features and Some Life Style Factors of Multiple Sclerosis Patients in North of Khuzestan a
In our study, female patients were 96 (67.6%), male patients 46 (32.4%), and female/male ratio was 2.08:1, but in younger patients the sex ratio was less, so that in below the age of 20 this proportion was 1.3:1. Mean age of the patients was 33.4 ± 9.4. Moreover, 70% of them were 20 - 39 years old. The mean age of both sexes was equal (Table 2). Mean age at onset was 25 ± 8 and 70% of patients had 10-29 years old at the beginning of the disease (Table 3). In addition, 70% of the patients had no history of immigration while 16% had a history of immigration before 16 years old and 14% after it. Almost half of the patients were belonged to the crowded families with more than six children.
| Mean Age, y | Lowest Age, y | Highest Age, y | |
|---|---|---|---|
| Female | 33.56 | 13 | 54 |
| Male | 33.15 | 13 | 51 |
Mean Age of Patients in Both Sexes
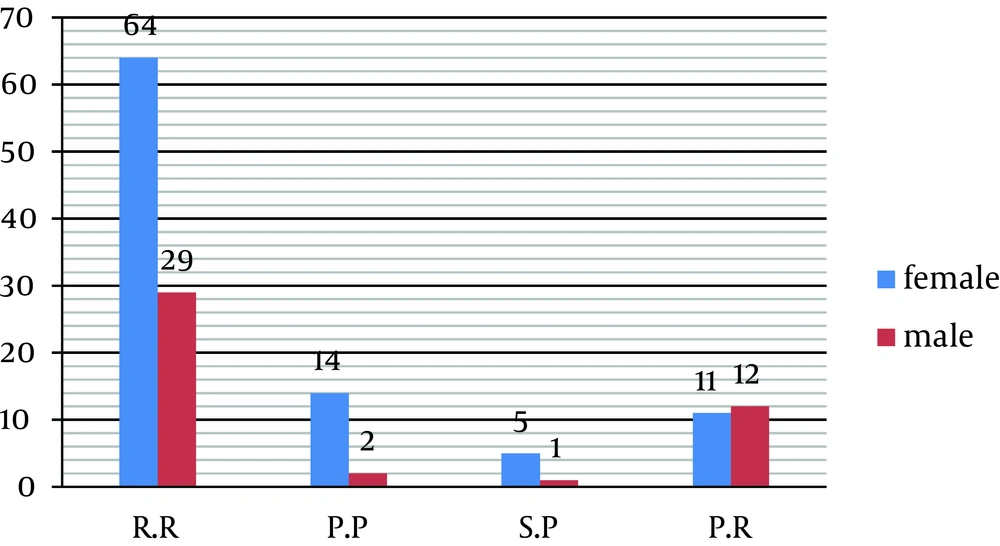
Relapsing- remitting (RR), secondary progressive (SP), primary progressive (PP) and progressive-relapsing (PR) were seen respectively in 69.5%, 4.2%, 11.3%, and 15% of the patients (Figure 2). In addition, 93% of the patients were nonsmokers. Findings showed that there is no significant difference between sex and subtypes of disease (P = 0.08) (Tables 4 and 5). On the other hand, clinical feature of the disease in women was the same as men.
| Number of Patients | Mean ± SD | |
|---|---|---|
| Mean age at onset | 142 | 25.2 ± 8.9 |
| Mean age of diagnosis | 142 | 26.8 ± 9.09 |
Mean Age at Onset, and Mean Age of Diagnosis in Multiple Sclerosis Patients of North Khuzestan
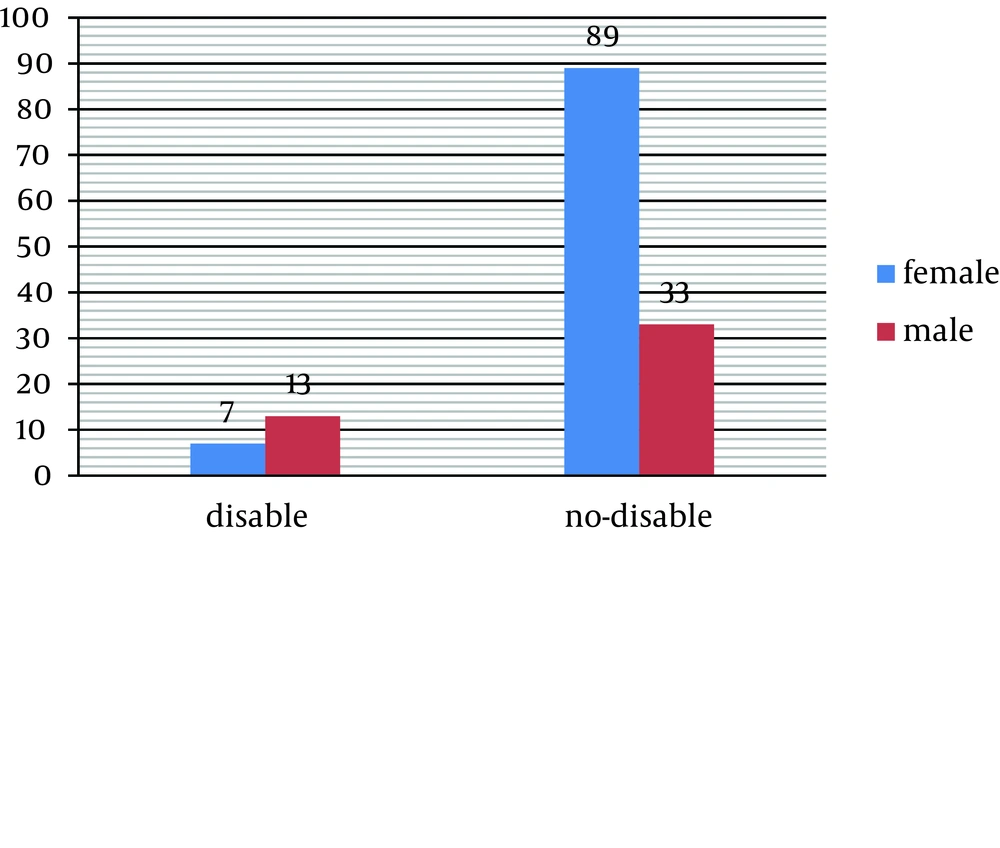
The initial symptoms of 36% of the patients were paresthesia and limb numbness, 32.4% had optic neuritis, 27.5% cerebellar disorder and ataxia, 2% had tremor, muscle weakness was seen in 20% and 32.4% had urine disorders together with other abnormalities (Table 6). The findings of this study did not show a significant relationship between demographic characteristics like sex, marriage, level of education, living place (town/village) and clinical course and also signs at onset. There was a significant correlation between sex and severe disability and more than 50% of the disabled patients were men (P = 0.02) (Table 7) (Figure 3).
| Sign | Female | Male | Total |
|---|---|---|---|
| Paresthesia | 38 (39.6) | 13 (28.3) | 51 (35.9) |
| Limb numbness | 8 (8.3) | 6 (13) | 14 (9.9) |
| Diplopia | 17 (17.7) | 9 (19.6) | 26 (18.3) |
| Darkness eye | 31 (32.3) | 15 (32.6) | 46 (32.4) |
| Ataxia | 24 (25) | 15 (32.6) | 39 (27.5) |
| Urinary incontinence | 12 (12.5) | 6 (13) | 18 (12.7) |
| Tremor | 0 (0) | 2 (4.3) | 2 (1.4) |
| Motor deficit | 7 (8) | 20 (43) | 27 (20) |
Initial Signs at Onset in Patients With Multiple Sclerosis in North Khuzestan a
| Value | Degrees of Freedom | P Value | |
|---|---|---|---|
| Pearson Chi-square | 5.432 a | 1 | 0.02 |
| Number of valid cases | 142 |
Correlation Between Sex and Disability
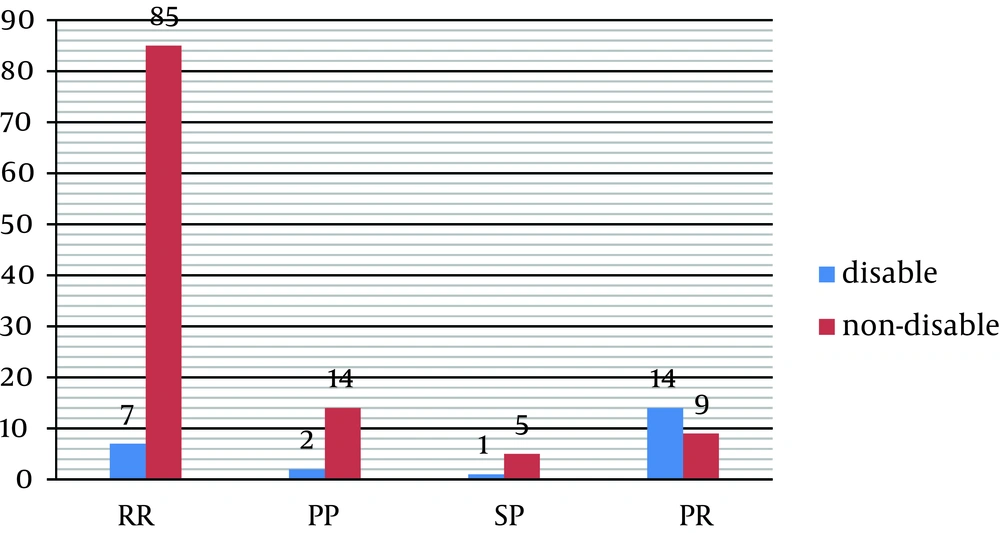
Furthermore, a statistically significant correlation was found between subtypes of MS and disability so that about 70% of disabled patients belong to the PR subtype (P = 0.001) (Table 8, Figure 4).
| Value | Degrees of Freedom | P Value | |
|---|---|---|---|
| Pearson chi- square | 20.449 a | 5 | 0.001 |
| No. of valid cases | 142 |
Correlation Between Disability and Clinical Course of Multiple Sclerosis
5. Discussion
The prevalence of MS in the north cities of Khuzestan Province was 15 per 100,000 people and because some cases might not recognize in the study; therefore, the prevalence is probably higher than this. In a recent study by Sharafaddinzadeh et al. (17) the prevalence of MS in Khuzestan was 18.50 per 100,000 people. The incidence of MS in different population is 1.5-30 per 100,000 people each year, and in some regions it was 150 - 250 (3, 14, 17). The world health organization (WHO) reported that in Asia the prevalence of MS is 4 per 100,000 people (18). In other studies from different cities in the center and north of Iran, the prevalence of MS was reported 35.5 per 100,000 people, and 20 per 100,000 (19). Multiple sclerosis is more common in people who live far from the equator (11). Other researchers reported that decreased sunlight exposure has been linked with a higher risk of MS. Decreased vitamin D production and intake as a main biological mechanism used to explain the higher risk among those less exposed to sun (20). Therefore, the higher level of MS risk in the center and north than the south of Iran (Khuzestan) seems reasonable. A two times higher prevalence of MS was found among married people, both the women and men than single patients. As a weakness of our study, we did not know whether the disease happened before the marriage or after. Moreover, there are few studies concerning the role of marriage status in MS (21). Recent study showed that 78% of individuals with MS had a diploma or lower rate of education and 81% of them were housekeeper and unemployed. About half of the patients were living in crowded families with more than six children. The income of 64% of the patient’s family was ≥ 6000000 Rial (IRR), while that 19% had 6000000-9000000 IRR and the income of 17% was ≤ 9000000 IRR. On the other hand, most of the patients had low or medium socio-economic status. In a report from America, there was a significant relationship between the socio-economic status and occurrence of MS, so that high literacy and well socio-economic conditions are more susceptible to MS (22). In another report, the poor economy has a direct role with the occurrence of MS (14); however, opposite results in different studies needs to broad investigations in larger population. In our study, patients were 96 females (67.6%) and 46 males (32.5%), and the female/male ratio was 2.08:1. According to the report of the national hygienic center of America in 2001, the sex ratio was 2.6:1 and in Hong-Kong was 3.2:1. A broad study on 27000 patients with MS in Canada showed that the female/male ratio has been increased for at least 50 years from 1.9:1 to 3.2:1. The findings of a study in Khuzestan, showed that female/male ratio was 3.1:1. Some reports in Iran announced the rate of 1.2:1 and 1.5:1 in 1999, and during the year of 2003, other studies showed the female/male ratio of 2.5:1 and 2.6:1 (4, 14, 17, 18, 23). A recent study on Swedish, MS patients showed that the female/male ratio was 2.42:1 that is close to our results (24). Of course the estimated female to male ratio in our study was less than other Iranian reports and some other places of the world. The environmental factors causing the shift in sex ratio were attributed to changes in lifestyle factors of women. These include higher numbers and changing roles of women in the workforce, outdoor activities, dietary habits, and alterations in menarche and in the timing of childbearing years, in addition to premature mortality of men compared with women. Besides, the sex ratio findings are the result of a compensatory decrease in incidence among men, differences in time to diagnosis and age of onset could potentially affect the sex ratio in the short term if a relative delay was sex specific (4). Some specialists argued that estrogen strongly affects the sex ratio of spontaneous autoimmunity and their role in MS and other autoimmune diseases has been widely accepted; however, oral contraceptives do not increase risk of the disease (25). Findings showed that as age of patients become younger, the sex ratio proportion is less, so that in patients with below 20 years old, the ratio was 1.3:1 and in above this age, ratio was 2.08:1, which is probably due to the lower age of onset in the recent years. Orton et al. (4) reported a significant relationship between age and sex ratio, so that in younger patients the ratio was increased. Of course this is inconsistent with our findings. In the early 1900, the sex ratio in MS was reported by several researchers to be close to unity and evenly men were more affected than women in the ratio of 3:2 (26, 27). In recent years, the female/male ratio had decreased, which seems due to the effect of environmental factors (4). According to this study, mean age of patients was 33.4 ± 9.4 and most of them had 20 - 39 years old and there was no significant difference in the mean ages between both sexes. In a study in Khuzestan, mean age was 31.4 ± 8.50 years that is similar to our study (17). It seems that MS mostly occurs in adults, and rarely in childhood. In our study, 70% of patient’s age of onset was 10 - 29 and the mean age of onset was 25 ± 8 years old and mean age of diagnosis was 26 ± 9. These results is in-line with those of many other investigations (28-30). It is probably that early diagnosis of MS is due to public awareness; faster refer of patients for a definite diagnosis and development of diagnostic equipment. Data showed that RR in 69.5%, PR in 15%, PP in 11.3%, and SP in 4.2% of the patients were seen. According to the classification of the United State national multiple sclerosis society, 85% - 90% of the patients have the RR subtype, and PP in 10% - 15% of the individuals with MS is seeing (8). Yousefi Pour and Rasekhi (14) found that, RR was seen in 80% - 83% of the cases. In other studies the PR and SP had rates of 7% and 5%, respectively (16, 23). Our study showed considerably increased rate of PR (15%) that needs more evaluation. This subtype of MS has a poor prognosis and at the time of onset, patients would experience progressively decline of their physical abilities. In addition, about 70% of the disabled patients in our study were from this subtype, which is reasonable. In the recent study, the first presenting sign in 36% of the patients was sensory deficits, while visual impairment, cerebellar disorders, motor defect, and urinary dysfunction with other deficits simultaneously were the first symptoms of 32.4%, 27.5%, 20% and 32.4% of the patients, respectively. No statistically significant relationship was found between sex and signs at onset. The findings of the other studies in this manner is similar to our study so that in a study in Iran, paresthesia was the most common symptom at onset (46% of cases), visual impairment and motor deficit were reported respectively in 33% and 26% of the cases (15). In a study by Maghzi et al. (29) the most common presenting symptoms were sensory signs and optic nerve involvement (78.2%), followed by cerebellar symptoms (11.3%) and motor deficit (10.3%). In a study in Saudi Arabia only 19% of the patients had visual impairment at onset while paresthesia and motor deficit were reported in 43%, 15% of the cases, respectively (31). It seems that the signs and symptoms of the disease at the onset of MS were almost like to those which were reported in other regions, and because the initial signs can affect the prognosis, so it must be considered. Findings of our study showed that there was a statistically significant correlation between sex and disabled patients so that 55% of paralyzed patients were men. In a recent study in a Swedish population Anens et al. (24) reported that men with MS were less physically active, in addition to more limitations in activities and walking ability than women, probably a result of being more physically affected by the disease. This finding is in accordance with our results that men had more difficulty in ambulatory activities than women. However, Motl et al. (32) reported that there was no significant difference in physical activity and sex. Because few studies have been focusing on gender and physical activity, future studies are of value. Because of variations in the prevalence of different studies in Iran and Asia, this is necessary to do more extensive researches in different regions, to determine the high risk places and recognize the susceptible environmental factors with respect for differences. In this study female to male ratio was lower than other studies in Iran. Our analysis was not without potential confounders, although findings showed that the sex ratio in MS is varied and recent studies imply that the female/male ratio is decreasing, which needs more studies to access the role of environmental factors. The other finding of this study was the negative role of socio-economic factors on MS that needs more investigation. Moreover, an increasing rate of the PR subtype among patients and severe disability that was seen in men more than women were noticeable.