1. Background
The hypothalamic-pituitary-adrenal (HPA) system regulating plasma corticosterone levels, controls many physiological processes under basal physiological conditions or during stress (1-3). In this regard, many articles reported that dysregulation of the HPA axis is involved in depressive disorders, and reflected primarily in excessive secretion of natural glucocorticoids (4-7). On the other hand synthetic glucocorticoids such as Dexamethasone (9 α-fluoro-16α-methylprednisolone) that is a potent and rather selective glucocorticoid receptor (GR) ligand has direct effects on energy metabolism, the immune system and HPA axis activity and also if moderate amounts of dexamethasone are injected (8), the pituitary will be the preferred location for the inhibitory activity of DEX at the HPA axis (8-10) because the efflux transporter P-glycoprotein regulate HPA axis function limits the access of synthetic glucocorticoids to the brain and there is almost no P-glycoprotein in pituitary (10).
Increasing depressive-like behavior is reported in zinc-deficient animals, probably through stimulating HPA system (11), since abnormal glucocorticoid secretion is shown along with zinc deficiency (12-14). Interestingly, Zn-containing neurons are mostly found in the areas known to be important in anxiety/depression including cerebral cortical regions, hippocampus, most amygdaloid nuclei, and the lateral septum (11). In case of interaction between zinc and glucocorticoid, different results are reported.
In addition, normalization of low levels of serum zinc and high levels of corticosterone in zinc deficient rats with antidepressant therapy is reported (15). Furthermore, zinc is active in forced swimming test (FST) and tail suspension tests (TST) in mice and rats, as well as increases efficacy of conventional antidepressants (16, 17).
It is evident that zinc and glutamate co-released from zincergic neurons, and corticosterone dose dependently increase the release of reactive zinc from neuron terminals in the hippocampus; thus, glucocorticoid-glutamatergic interactions can be a potential model to describe the events that occur in stressful situations (18), and glucocorticoids by homeostasis of synaptic Zn2+ and glutamate have important role in behavioral responses (19).
Considering that rats deprived from sufficient amounts of zinc, showed an enlargement of the adrenal gland (20) and an increase in serum corticosterone levels, it can be concluded that zinc deficiency affects dysregulation of the HPA axis (11, 21, 22). Furthermore, systemic injection of zinc shows antidepressant effects in male rats (23).
2. Objectives
Furthermore, considering the genomic and non-genomic interactions between central GRs and NMDA receptors in other fields such neuropathic pain in rat (24) and according to the existence of positive correlation between zinc deficiency and serum glucocorticoids (11, 21, 22), and since the improvement of neuropsychological performance occurred with zinc supplementation (11, 20, 22), the current study aimed to investigate the possible interaction between zinc chloride and synthetic glucocorticoid dexamethasone on the depressive like behavior using a FST in adult male rats.
3. Materials and Methods
3.1. Animals
One-hundred and four male Wistar rats (200 ± 20 g), three months old, were housed in plexiglass cages (four animal per cage) at a temperature of 23 ± 2°C and a 12/12 hours light-dark cycle (light on at 07:00). All animals were provided by Ahvaz University of Medical Sciences animal breeding house and were kept for seven days before starting the experiments to adapt to the new houses with free access to food and water. Then, they were divided into 13 groups. Vehicle (saline) was administrated in the intact and control groups and the other groups were administrated with dexamethasone sodium-phosphate and ZnCl2 in different doses. All experiments were performed between 8:00 am to 2:00 pm, and were according to the instructions to work with laboratory animals approved by Shahid Chamran University of Ahvaz.
3.2. Apparatus
The experimental device used to measure depressive-like behavior was a plexiglass cylinder (height 50 cm, diameter 30 cm) containing 30 cm of water, maintained at 25°C was used as FST apparatus (25).
3.3. Drugs
The following drugs were used: Dexamethasone sodium-phosphate from Aburaihan Pharmaceutical Company (Iran) and zinc chloride from Merk (Germany). All drugs were dissolved in the saline and volume of all injections was 1 mL/kg.
In the first experiment, animals were subcutaneously injected with different doses of DEX 24 hours before evaluation of depression (25). In the second experiment animals received different doses of ZnCl2, intraperitoneally (IP) 24 hours and 30 minutes before evaluation of the test, and in the third experiment the effects of different doses of ZnCl2 (10 and 20 mg/kg) + DEX (1 mg/kg) pretreatment groups were examined in the FST, and the total immobility time and latency to immobility were recorded as measures of depression.
3.4. Procedure
Rats were divided into 13 groups (n = 8 each) including: non treated, saline, DEX (0.5, 1, 5, 10 and 30 mg/kg, SC) (3, 25) and ZnCl2 (2.5, 5, 10 and 20 mg/kg, IP) (26). The doses tested for interaction groups were ZnCl2 (10 and 20 mg/kg, IP) and DEX (1 mg/kg SC) pretreatment.
3.5. Statistical Analysis
The collected data were analyzed using one-way analysis of variance (ANOVA). Post hoc analysis using the least significant difference (LSD) was applied when appropriate. The decision criterion for statistical tests of significance was P < 0.05.
4. Results
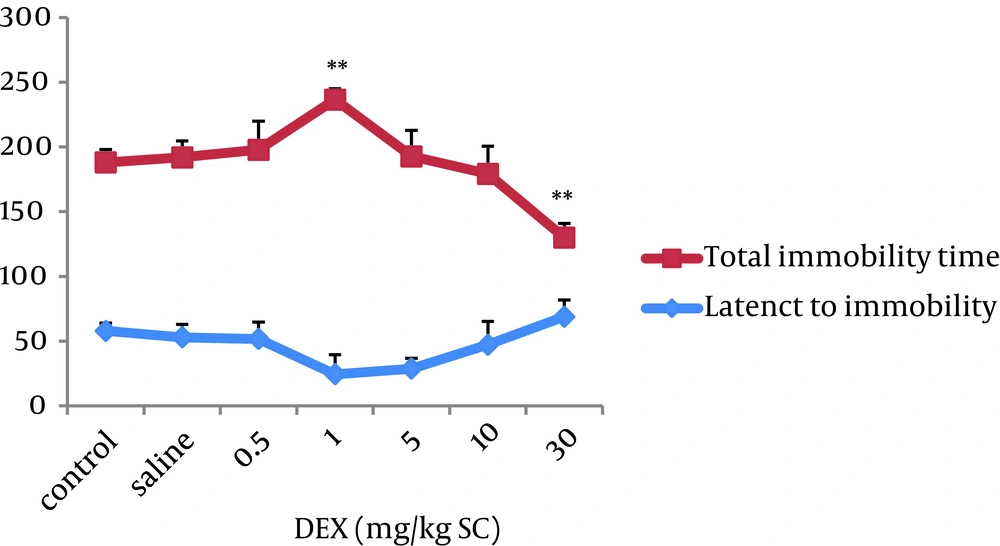
The first experiment examined the effect of DEX on depression. Results showed that DEX 1 significantly increased while DEX 30 decreased total immobility time compared with those of saline group respectively (P < 0.01). Further comparisons revealed a decrease of latency to immobility in DEX 1 as compared to those of saline group, but did not reach significance criteria (Figure 1).
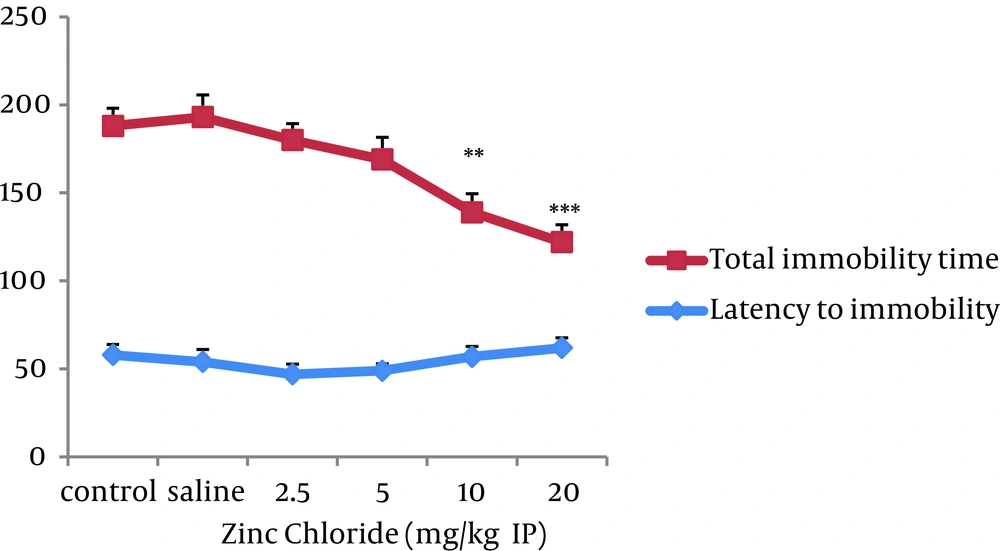
The second experiment examined the effect of zinc administration on depression. Results showed a significant decrease of total immobility time in the groups treated with zinc 10 mg/kg (P < 0.01) and zinc 20 mg/kg (P < 0.001) as compared to that of saline group. There was no significant difference in latency to immobility among all groups (Figure 2).
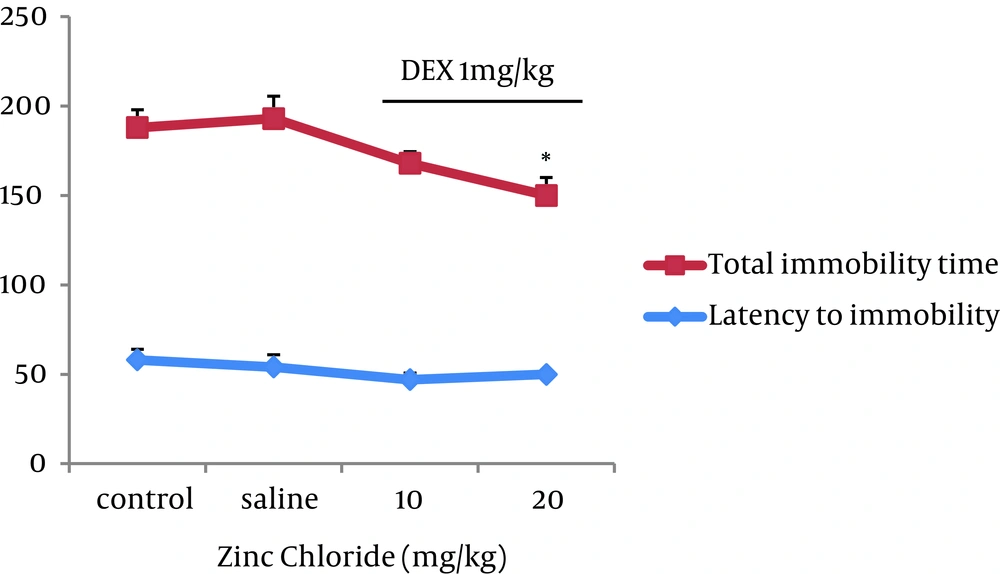
The third experiment examined the effect of zinc on depression in groups pre-treated with DEX 1. There were significant decreases (P < 0.05) of total immobility time in the groups that received zinc (20 mg/kg) + DEX (1 mg/kg) concomitantly as compared to that of the saline group. There was no significant difference in latency to immobility among groups co-administered with zinc + DEX1 (Figure 3).
5. Discussion
The current study investigated the effects of dexamethasone, zinc and their interaction on depression assessed by FST. The findings indicated that ZnCl2-treated groups showed a gradual and dose dependent decrease in depressive-like behavior. DEX had a dual effect on depression, increased and decreased depression with lower and higher doses, respectively. Effective dose of zinc (20 mg/kg) significantly decreased dexamethasone induced depressant effects in rats. Therefore, it may reasonably be speculated that zinc supplementation has potential importance as antidepressant therapy in rats.
The obtained results were interesting and the authors found some possible explanation to indicate the mechanisms underlying DEX effects on depressive-like behavior. The current study suggested that dexamethasone has dual effects on depression; therefore, a low dose of DEX showed an increased level of depression while higher doses had antidepressant effects (25).
It seems that low doses of dexamethasone have little access to the brain, probably due to the P-gp pump activity in the blood-brain barrier (8, 27). Poorly binding of the small amount of dexamethasone (unlike cortisol) to minralocorticoid receptors (MRs), left these receptors without ligand in the brain. Deprivation of this part of brain from corticosteroid (CR) signaling (28), and creation of hypocorticoid brain state (8) result in a number of changes in neurotransmitter systems and is sensed by central regulatory elements of the HPA system (28, 29). Thus divergence of central hypocorticoid state due to hampered access of low doses of dexamethasone (8), followed by peripheral hypercorticoid state or increased cortisol circulation (8, 30, 31) would result in HPA dysregulation and development of depression in rats (5-7). However, the P-glycoprotein barrier is involved and can be overcome by high amounts of dexamethasone to suppress the HP axis activation and decrease the depression symptoms in rats (8). Following the above mentioned descriptions, some articles reported that higher doses of dexamethasone had much more inhibitory effects than lower doses on HPA axis function in humans (27, 28, 32).
Li et al. reported that neonatal DEX exposure showed behavioral disorders such as anxiety and depressive-like behavior in juvenile and adult mice and interestingly, pretreatment with Ro 63-1908 (an antagonist at GluN2B subunit) or L701324, antagonist of the glycine modulatory site on the NMDA receptor significantly decreased these behavioral abnormalities through altering NMDA receptor neurotransmission in the hippocampus (33). Previous studies also showed that preincubation of hippocampal neurons with high levels of corticosterone or dexamethasone for 20 minutes induced a rapid and non-genomic Ca2+ elevation under NMDA stimulation (34).
In other words, dexamethasone exerts its rapid neurotoxic effects probably by increasing calcium flow through NMDA channel, which is blocked by NMDA receptor antagonists (35).
Further support is provided when materials that decrease NMDA activation have antidepressant effects in clinical studies (36). Increased glucocorticoid levels as a result of clinical situations including depression, the Cushing syndrome, and injection of synthetic glucocorticoids can induce behavioral disorders that may occur through NMDA receptor downstream events.
Although many studies revealed that dexamethasone potentiates glutamate neurotoxicity and enhanced expression of NMDA sub unit genes and proteins, an article reported that dexamethasone and corticosterone decrease calcium overload in neurons and astrocytes and can be useful to protect glutamate cell toxicity (37). In an overall view, it is evident that there is a possible correlation between the activation of corticosteroids and NMDA receptors, which may result in depressive behaviors.
Thus, in future, NMDA receptor antagonist may have a potentially therapeutic role in the behavioral disorders (38), and zinc as a potent inhibitor of NMDA receptor could be a major focus of administration as an antidepressant agent (36). Also it was shown that chronic antidepressant therapy increased concentration and potency of zinc in blocking NMDA in the Central Nervous System (39).
For instance, acute, sub-chronic and chronic administration of 65 mg/kg zinc hydroaspartate (11.5 mg Zn/kg) was effective in the FST and decreased total immobility time in rats (40). In another report sub-chronic or chronic zinc administrations were more effective than acute dose in the FST (41).
Another study showed that zinc chloride (10 and 30 mg/kg; P.O) reduced immobility time in the TST and had synergistic antidepressant effects in sub-effective doses (42). There are some reports in which zinc had no significant effect on depression. For example, Franco et al. reported that acute administration of zinc chloride (5, 15 and 30 mg/kg, IP) did not change immobility time compared with the control group. Also, animals receiving single zinc dose (5, 15 and 30 mg/kg, IP) 24 hours prior to analysis showed no changes in the FST (43).
It is speculated that the observed effects of dexamethasone in the current study might be explained in terms of interference with neurotransmitter systems, particularly the glutamatergic system, which is also affected by zinc. Interestingly, these effects were observed after the short period of time that had elapsed between the injections and the test, suggesting a nongenomic effect via membrane-located receptors (44).
For example corticosterone and dexamethasone increased extracellular aspartate and glutamate levels in the CA1 area of the hippocampus (45), while zinc as a negative feedback factor decreased glutamate release in the hippocampus (46-48).
Also it seems that the nitric oxide system performance following NMDA receptor activation may have a role in the interference of zinc chloride and dexamethasone in the modulation of depression. Since NMDA receptor antagonists (49, 50) and nitric oxide synthase inhibitors affected depression (49, 51) by decreasing plasma corticosterone levels (52), it is reported that zinc as a potent endogenous inhibitor of the NMDA receptor and nitric oxide synthase in the brain, has antidepressant action both in the FST and TST. Rosa et al. showed that acute administration of zinc chloride in 30 mg/kg IP significantly decreased immobility time in the forced swimming test (36).
But dexamethasone is argued to enhance NMDA excitotoxicity in seven-day-old rats, time dependently (45). In conclusion, the current study suggests that dexamethasone and zinc chloride affect the modulation of depressive-like behaviors and zinc chloride can partially reverse depressive effects of dexamethasone in adult male rats, as assessed in the forced swimming test.