1. Background
Leptin, a product of Ob gene, is a newly discovered single-chain prohormone with the molecular mass of 16 kDa, which seems to play a key role in regulating body weight (1). Leptin is produced by various adipose tissues, although other tissues like fundus of the stomach, skeletal muscles, liver, placenta (2), heart (3), cumulus oophorus and granular cells of human ovary, mammary gland (4), and epithelial cells of the stomach (5) produce it too. This hormone is the main peptide secreted from the adipose tissue; its serum concentration is an important index in the regulation of food intake and energy consumption. Various factors might influence gene expression and plasma leptin level, including exercise and physical activities (6). The effects of physical activity on the leptin level have recently been a hotly debated issue. The reports on the leptin reaction to the physical activity are confusing. The majority of the research indicates a decrease or lack of change in leptin level in reaction to short periods of physical activity (one session) (7). Decrease in the level of leptin in male athletes can be expected after two separate exercise tests in which the level of energy consumption is 800 to 1500 kcal. The decrease in plasma leptin occurs 48 hours after the exercise and following the decrease in the insulin level (8). Karamouzis et al. (9) measured the level of leptin among swimmers in a 25000 meters swimming competition event. In this study, the 16 swimmers swam the distance in the average time of 8.5 hours. The results indicated a significant reduction in the subjects’ leptin level. Elias et al. (10) observed a decrease in the leptin level during an incremental exercise task which continued to the level of exhaustion. Olive and Miller (11) stated that 60 minutes of exercise with the maximum oxygen consumption of 70% significantly reduces the level of leptin in men. Bouassida et al. (12) attributed the leptin reduction after exercise to the consumed calorie and the duration of the exercise. Hickey and Perusse showed, on the other hand, that leptin plasma levels do not change as a result of a single short-time exercise session (13, 14). A review study revealed that short periods of exercise (less than 60 minutes) and exercises in which the consumed energy is less than 800 cal, did not change the leptin level (7). More research on the possible impacts of hormones and metabolic materials on the leptin levels is needed in order to understand why in some cases exercise leads to the reduction of leptin level and in other cases does not (15).
Leptin enhances the oxidation of fat and reduces triglyceride in skeletal muscles. In a short period of time, leptin is controlled by creating a negative energy balance, through diet and exercise, which leads to the reduction of leptin level. In contrast, the positive energy balance increases leptin levels. Glucose is the most abundant monosaccharide absorbed by the human body, comprising more than 80% of the carbohydrate energy absorbed. Glycolysis blockers, or factors preventing glucose transfer into fat cells, might have a significant role in the reduction of leptin metabolism, indicating increase in the leptin level requires glucose absorption and metabolism in the cell (16). That leptin production occurs after the increase of insulin in reaction to the rise of glucose (because of food intake) has been proved. Also, during fasting, when insulin is reduced, the leptin concentration decreases (17). Therefore, the amount of glucose in the blood can explain the leptin level. In one of the few studies conducted on this subject, Jurimae et al. (18) investigated the effect of one session boating exercise on the leptin level in male students. In this 30-minute exercise, 13 male rowers rowed a distance of 7870 meters. Results of this study indicated that the concentrations of leptin and glucose in the blood decrease as a consequence of the exercise. The researchers concluded that the leptin level was sensitive to this short time exercise because all large muscles were involved (18).
2. Objectives
Given the contradictory results regarding the change in the leptin level after a single exercise session and the findings of Jurimae et al. (18) with respect to the reduction of glucose and leptin level, this study set out to answer these questions:
Are there any significant differences in the plasma levels of glucose and leptin after one session of 3000-meter swimming exercise under two continuous and intermittent conditions?
Is there any correlation between leptin and glucose level?
3. Materials and Methods
A total of 24 male elite swimmers (18 - 30 years, average age: 25.33 years, SD: 3.54; average weight: 76.99 kg, SD: 8.27; average height: 180.29 cm, SD: 6.003) participated in this study. They had no prior information with the test and all gave their informed consent before participating in the study. Inclusion criteria were as follows: being elite swimmers, 18 to 31 years, residing in Ahvaz city, and having no particular diseases. Exclusion criteria were as follows: opting in research, leaving the study, and contracting infectious disease.
Participants were randomly divided to two groups: training and control groups (12 participants in each group). Study design consisted of pretest, training phase, and posttest phase (immediately after and 24 hours after training). Participants of training group took part in two training conditions (continuous and intermittent). At first, they swam continuously 3000 meters with 50% - 60% of maximum heart rate in the standard pool. After 10 days, same participants swam intermittently 3000 meters (i.e. 15 × 200 meter with 30 second rest in between) with 70% - 80% of maximum heart rate. During the training period, the control group was present without any activity in the pool.
The subjects were provided with a list of food that could affect the results of the study and were asked to lower their intake of these foods to the lowest level during the study. All the subjects continued with their usual eating program during the study. They did not perform any physical activity 25 hours before and after the test. Blood samples were collected from the training and control groups at three different occasions (before the test, immediately after, and 24 hours after the test). The blood samples were taken to the laboratory and after being centrifuged at the laboratory temperature, were frozen to be kept at -70°C. The serum leptin was then measured using ELISA method and Mediagonest model kit. The level of glucose in the subjects’ blood was also measured during pretest and immediately after the exercise.
3.1. Statistical Analyses
First, we checked the normality of the data as well as the homoscedasticity. The sphericity assumption was also tested using the Mauchly’s test of sphericity. Data showed that the normality and sphericity were not violated and thus parametrical statistical tests could be used. A within group variance with repeated measures was used to indicate exercise effect in continuous and intermittent training for serum leptin. The paired t test was used to indicate training effect on glucose amount. One-way ANOVA was used to compare serum leptin and glucose amounts on the pretest to ensure the 3 groups were not different before the exercise. One-way ANOVA was also used to compare serum leptin and glucose amount at posttest. Finally, we used Pearson correlation coefficient to examine the relationship between serum glucose and leptin levels.
4. Results
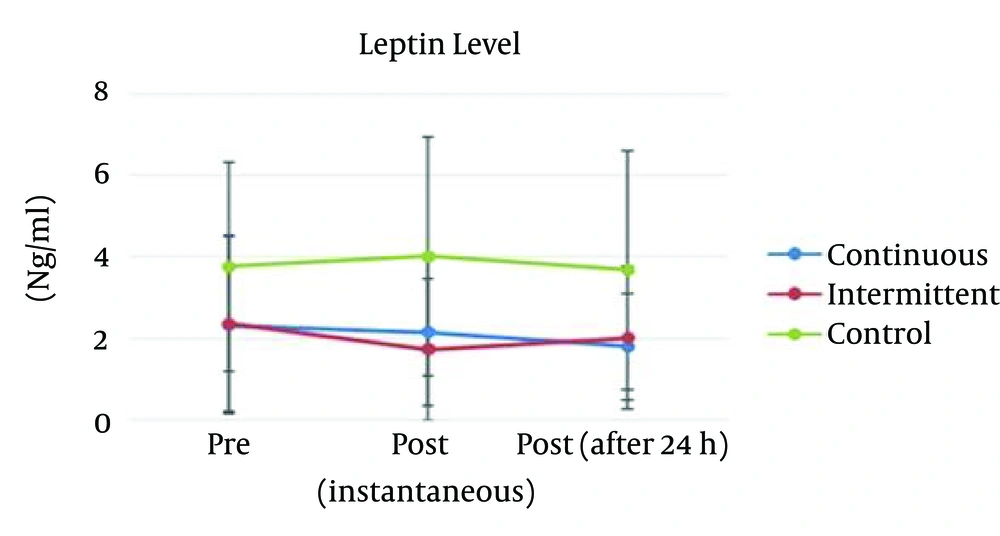
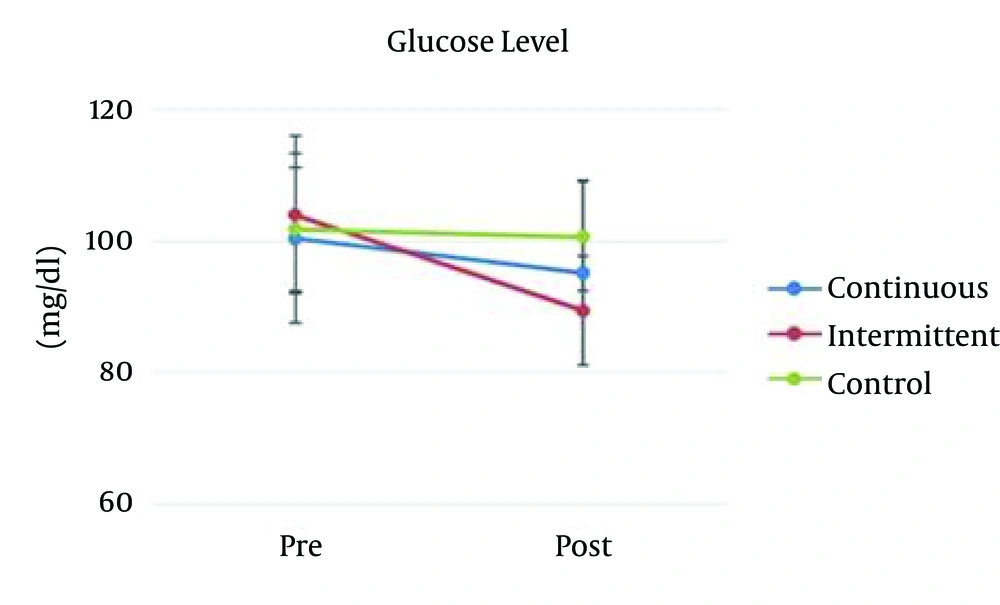
To test for groups’ differences (i.e., continuous, intermittent, and control) with regard to dependent variables (i.e., serum leptin, and glucose) in the pretest phase, 1-way analysis of variance (ANOVA) was utilized. Results indicated that the groups were similar at the pretest phase regarding serum leptin: F (2, 35) = 1.49, P = 0.23 (Figure 1), and glucose amount: F (2, 35) = 0.29, P = 0.74 (Figure 2). The serum leptin and glucose rates in continuous, intermittent, and control groups during pretest and posttest phases are presented in Table 1.
| Variable | Group | Pretest | Posttest | |
|---|---|---|---|---|
| Immediately After Exercise | 24 Hours After Exercise | |||
| Leptin, Ng/mL | ||||
| Continuous | 2.33 ± 2.17 | 2.16 ± 1.79 | 1.81 ± 1.30 | |
| Intermittent | 2.37 ± 2.16 | 1.74 ± 1.74 | 2.02 ± 1.75 | |
| Control | 3.76 ± 2.56 | 4.02 ± 2.93 | 3.68 ± 2.92 | |
| Glucose, mg/dL | ||||
| Continuous | 100.41 ± 12.81 | 95.16 ± 14.08 | - | |
| Intermittent | 104 ± 12.03 | 89.50 ± 8.27 | - | |
| Control | 101.75 ± 9.32 | 100.66 ± 8.32 | - | |
4.1. Leptin
In continuous training, the results indicated that there were not significant differences among 3 conditions, F (2, 22) = 2.31, P = 0.12, and η2 = 0.09 (Figure 1).
In intermittent training, the results indicated that there were significant differences among 3 conditions, F (2, 22) = 3.24, P = 0.04, and η2 = 0.312. Bonferroni post hoc analysis indicated that there was significant difference between pretest and immediate posttest results (P = 0.004). But the pretest and 24 hours after posttest results were not significantly different (0.32). The post hoc analysis also indicated the immediate posttest and 24 hours after posttest results were not significantly different (0.27) (Figure 1).
The serum leptin amount in immediate posttest was analyzed using one way ANOVA. This analysis indicated a significant main effect among groups, F (2, 33) = 3.30, P = 0.04, and η2 = 0.167. Tukey-Kramer post hoc analysis indicated that there was significant difference between intermittent and control groups (P < 0.001). The post-hoc analysis also indicated the intermittent and continuous groups were significantly different (P = 0.007), but the continuous and control groups were not significantly different (P = 0.80). However, the serum leptin level at 24 hours after posttest was not significantly different (F (2, 22) = 2.83, P = 0.07, and η2 = 0.16).
4.2. Glucose
In continuous training, the result of paired t test indicated that there was not significant difference between pretest and posttest results; t (18) = 1.18, P = 0.26. In intermittent training, the result revealed that the pretest results were significantly different from posttest results; t (18) = 2.96, P = 0.01. The results of control group were not significantly different; t (18) = 0.61, P = 0.55 (Figure 2).
The glucose level in posttest was analyzed using one way ANOVA. This analysis indicated a significant main effect for groups, F (2, 33) = 3.33, P = 0.048, and η2 = 0.167. Tukey-Kramer post hoc analysis indicated that there was significant difference between intermittent and control groups (P = 0.037). However, the continuous and control (P = 0.42) and intermittent groups were not significantly different (P = 0.39).
4.3. Correlation
In order to investigate the relationship between leptin level and glucose amount, the bivariate correlations were computed. The Pearson correlation coefficient indicated statistically significant relationships between leptin level and glucose amount in immediate posttest (r = 0.26, P = 0.03) in intermittent condition, but there was not significant relationship between leptin level and glucose amount in immediate posttest (r = 0-.18, P = 0.56) in continuous condition.
5. Discussion
The main purpose of this study was to measure the effects of single session of continuous and intermittent exercise on the levels of leptin and glucose in the swimmers’ blood. The previous studies indicated that the level of leptin either does not change or decreases after a single session of exercise (13, 14). Jurimae et al. (18) argued that the observed lack of change in leptin level after single session exercise was due to the ignorance of the exercise intensity in healthy men and women. Another limitation of the studies indicating a lack of change after single session exercise was due to not involving all muscles in the exercise (7). Therefore, in this study, like the study conducted by Jurimae et al. (18), professional swimmers were chosen as subjects in order to make sure that they use all their muscles during the exercise. The results indicated that after one session of intermittent 3000-meter swimming the leptin level decreased significantly. There was also a reduction of leptin level 24 hours after the exercise, but this reduction was not statistically significant.
Research has shown that after the increase of insulin (in relation to food intake), the leptin level increases, and after the decrease of insulin during the fast, the leptin level decreases. Insulin-dependent transfer of glucose into the fat cells might explain the possible mechanism of insulin-induced secretion of leptin. Insulin carries the glucose into the fat cells through a glucose transporter protein, known as GLUT4. Then, the glucose acts as an intracellular signal, stimulating the secretion of leptin by fat cells. Therefore, the observed change in the concentration of serum leptin after one session of intermittent aerobic exercise in this study might be attributed to the changes in the insulin and glucose levels. Interestingly, the findings of this study confirm this argument, and also showed that there is a significant relationship between the leptin level and glucose level in the blood, and as the glucose level decreases, so does the leptin level. This finding is in line with the findings of Jurimae et al. (18) and Unal et al. (19).
A more significant factor might be the intensity and duration of the intermittent aerobic exercise. Bouassida et al. (20) argued that leptin reduction occurs after the physical activities in which the energy consumption is higher than 800 kcal. Therefore, the higher energy consumption during intermittent exercise might be one possible cause of leptin reduction; as such reduction did not occur after the continuous exercise in this study. No significant relation was observed between leptin level and glucose after continuous exercise, despite the results of the numerous studies that indicate the aerobic exercise change the glucose level. Perhaps, different reactions of insulin and glucose to the intensity and duration of continuous aerobic exercise have affected the level of blood glucose during the continuous exercise in this study, as the subjects performed the continuous aerobic exercise with 50% to 60% of the maximum heartbeat rate for an hour and the resultant reduction of glucose was not significant. The effect of the aerobic exercise on the cell and improvement of its sensitivity to insulin, facilitate the transfer of the glucose into the cell, and as a result the need for further secretion of insulin to increase the cell’s intake of glucose is reduced and pancreas produce less insulin. The main function of insulin is the reduction of glucose in blood circulation, but it also increases the cellular removal of the amino acids and the production of fat and protein, in their metabolism. Research has shown that during exercise the number of insulin receptors increases and their accessibility improves, and as a result the sensitivity of the body to insulin enhances. This phenomenon reduces the need for maintaining high levels of plasma insulin for transferring glucose into the muscular cells. In fact, the hormonal reaction is weakened after exercise (21).
Overall, the results of this study indicate that the intermittent exercise lowers the leptin plasma. In addition, it was revealed that this reduction is significantly related to the level of glucose in the blood. The continuous exercise was shown to have no effect on the plasma leptin level. Also, there was no relationship between the glucose and leptin levels regarding this type of exercise.