1. Background
Diabetes mellitus (DM) is known as a syndrome of defects in carbohydrate, protein, and lipid metabolism. Diabetes is one of the most prevalent abnormalities in all over the world. Diabetes mellitus is a disease characterized by hyperglycemia that is associated with inadequate insulin secretion or increasing tissue resistance to insulin. Type 1 DM occurs when pancreas failure is happen to secret enough insulin, and type 2 DM results from increasing insulin resistance and sometimes combination with relatively low insulin secretion (1, 2). Hyperglycemia causes different symptoms of diabetes, like polyuria, polyphagia, and polydipsia (3).
Diabetes and dehydration have a strong relation together. Hyperglycemia and ketoacidosis make osmotic dieresis and high body water loss, thus, they are at increased risk of dehydration and its complications (4). In addition, due to more water loss, patients with diabetes are usually more thirst than normal, so they need to drink more fluids to be hydrated. Cell hydration is important for insulin signaling and metabolic responses to other hormones. More insulin resistance in conditions like sepsis, diabetes and burn injury occurs due to low volume response of dehydrated cells to the insulin (5). Therefore, it is important to adequately hydrate diabetic patients with a kind of water with better hydration potential.
Water is created by hydrogen binding between one oxygen and two hydrogen atoms. There are many different structures in water based on how individual molecules bind together. Hexagonal structured water which is known as the most stable form of water, six individual H2O molecules held together by a hydrogen bind, which reshaped traditional usual structure. Hexagonal structure of water can be obtained with crossing normal water throw a magnetic field. Conventionally, many effects on aging process, cellular communication, enzyme function and other metabolic activities have been reported for magnetized hexagonal water (6).
Oxygen in magnetic hexagonal water formed in smaller cluster and supersaturated state. This structure makes a faster speed for passing throw stomach to the blood and increases cell membrane permeability. Hexagonal water can have a preventive effect on dementia and improve skin quality. It is said that microbes can't live in hexagonal water because of their disability in using the oxygen molecules of this structure and magnetic energy between hydrogen and oxygen. Also, hexagonal water has been associated with rapid hydration, better immune function, removal of toxin and better overall health condition (7).
2. Objectives
Due to few scientific studies presented about hexagonal water, this study aimed to evaluate the effect of hexagonal structure of water on glucose level and lipid profile in STZ-induced diabetic rats.
3. Materials and Methods
This experimental study was performed on 24 male Wistar rats (weighting 250 - 350 g) purchased from the animal house of Ahvaz Jundishapur University of Medical Sciences. Animal handling was performed with regard to Iranian Animal Ethics Society and local university rules. Animals were housed and maintained at 22°C under a 12-hour light/12-hour dark cycle, and had free access to water and standard pellet diet. They became diabetic by an intraperitoneal injection of STZ (40 mg/kg) (8) dissolved in sodium citrate buffer 0.1 M (pH = 4.2) just before injection. Diabetes was confirmed in fasting state of animals 4 days after injection by measurement of the tail vein blood glucose level with an accu-check sensor comfort glucometer (ACSCG). Rats with glucose level more than 250 mg/dL were selected as diabetic rats (all of them had glucose level more than 500 mg/dL). Afterwards, they were ran domly allocated into two groups and their blood glucose levels were measured prior, 10 and 20 days after the hexagonal administration from the tail vain with ACSCG. The test group received hexagonal water for 4 weeks and during this time control group received normal water.
To produce hexagonal water, magnetic field (near 10,000 gauss) was used. At first, normal water was filtered and the pollutants were removed and in the next step by using a strong gravity field, hexagonal structure was formed. At the end, some cube structure makers like Tourmaline and Argent (Ag) were added to water. Minerals were limited in about the range of 40 ppm. During the experiment, daily amount of water intake was measured. After 4 weeks, animals were fasted overnight and anesthetized with ketamine/xylasine and xylosin with 10/1 proportion and their blood samples collected from their heart into EDTA tubes and placed on ice. After centrifugation, plasma was collected and stored at 70°C.
Plasma glucose concentration was measured using the glucose oxidation method (Zistshimi, Tehran, Iran). Total cholesterol, triglyceride (TG) and high density lipoprotein (HDL) were spectrophotometrically measured by appropriate kits (Zistshimi, Tehran, Iran). Low density lipoprotein (LDL) and very low density lipoprotein (VLDL) levels were calculated by Friedwald (9, 10) formula as follows:


4. Results
Results were expressed as mean ± SD. Statistical analysis was performed with unpaired Student's t test (SPSS program v.11.5). P value < 0.05 was considered statistically significant. There was no mortality in any group throughout the experimental period.
4.1. Effect of Hexagonal Water on Blood Glucose Level
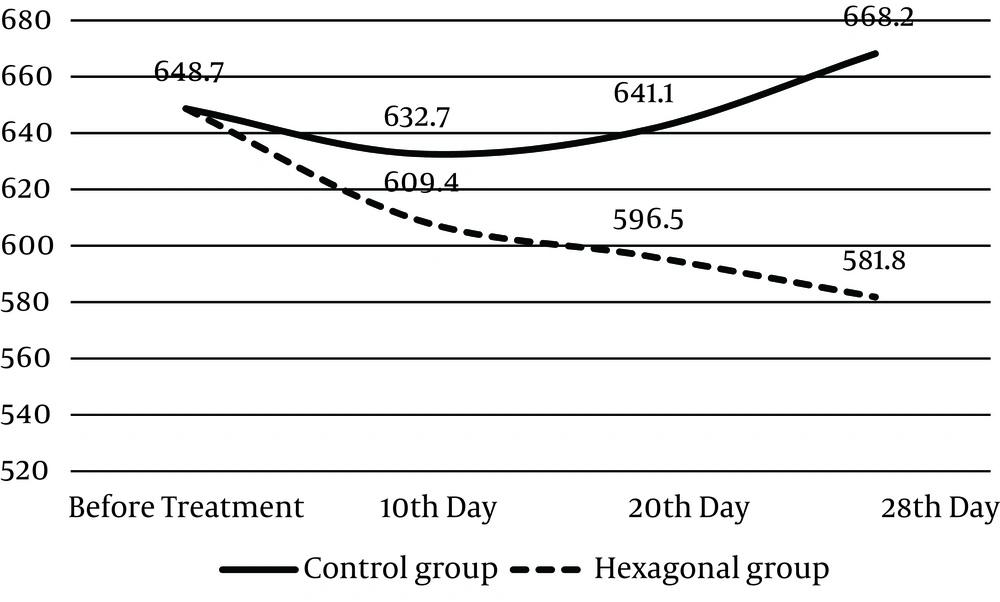
No significant differences was observed in the mean glucose levels between the hexagonal and control groups before treatment, 10 and 20 days after the administration of hexagonal water (648.7 ± 17.71, 609.4 ± 26.46, 596.5 ± 32.63 mg/dL vs. 648.7 ± 17.71, 632.7 ± 18.12, 641.1 ± 29.83 mg/dL, respectively); however, at the final day (the 28th day) a significant decrease was observed in blood glucose level of the hexagonal group compared with that of the control group (581.8 ± 34.48 mg/dL vs. 668.2 ± 21.24 mg/dL) (P < 0.05) Table 1 and Figure 1.
Diabetic rats were treated with normal or hexagonal water for indicated days and their blood glucose levels were measured at day 0, 10, 20, and 28. Data are shown as the mean ± SD of 12 rats/group.
| Variabels | Control Group, mg/dL | Hexagonal Group, mg/dL |
|---|---|---|
| Before Treatment | 648.7 ± 17.71 | 648. 7 ± 17.71 |
| 10th d | 632.7 ± 18.12 | 609.4 ± 26.46 |
| 20th d | 641.1 ± 29.83 | 596.5 ± 32.63 |
| 28th d | 668.2 ± 21.24 | 581.8 ± 34.48 |
Effect of Hexagonal Water on Blood Glucose Level
4.2. Effect of Hexagonal Water on Water Consumption
The mean water consumption in each rat in the hexagonal-treated group (154.3 ± 5.76 mL/day) was more than that of the control group (111.2 ± 5.51 mL/day) (P < 0.0001) Table 2.
| Group | Control Group, mL/d | Hexagonal Group, mL/d |
|---|---|---|
| Water consumption | 111.2 ± 5.51 | 154.3 ± 5.76 |
Effect of Hexagonal Water on Water Consumption
4.3. Effect of Hexagonal Water on Lipid Profile
Triglyceride (TG) level in the test group showed a significant decrease compared with the control rats (76.25 ± 6.76 mg/dL vs. 156.4 ± 19.88 mg/dL) (P < 0.01 ); however, in the test group, decrease in the serum cholesterol (Chol) and LDL levels and an increase in HDL level were not significant when compared with the control group. (64 ± 1.95 mg/dL, 51.58±5.91 mg/dL, 33 ± 2.01 mg/dL vs. 66.42 ± 3.59 mg/dL , 67.56 ± 6.17 mg/dL , 31.83 ± 1.84 mg/dL, respectively) (P > 0.05) Table 3.
5. Discussion
Patients with severe hyperglycemia are presented usually with sign of dehydration and metabolic derangement. Fluid loss in these patients is about 10% of body weight and loss of electrolytes such as sodium, potassium chloride, magnesium, and calcium are present in them. Their emergent treatment is the replacement of volume deficits and correction of their electrolytes. Dehydration in ketoacidotic and nonketoacidotic hyperglycemia is accompanied by elevation in counter regulatory hormones such as catecholamines, cortisol, and growth hormone, which increase serum glucose level (11-13). Rehydration of hyperglycemic patient can reduce insulin resistance and decrease releasing of counter regulatory hormones, thus, it can facilitate glucose transmission to the cells (14).
More water consumption in hexagonal-treated rats showed better hydration in comparison with the control group. It could be due to supersaturated state of oxygen molecules in hexagonal structure, which makes a faster transmission speed to the blood and cells (7).
Data showed that due to better hydration and its effect on insulin metabolism and counter regulatory hormones, the serum glucose level was decreased in the hexagonal group compared to the control group during the experiment period. Hyperlipidemia in diabetes is due to low clearance of lipids from blood because of the decreased lipoprotein lipase activity, which is dependent on the insulin sensitivity and sufficiency. The most important defect in insulin dependent diabetes is deficiency of lipoprotein lipase and in type II diabetes, the evidence of removal defect of lipids and overproduction of VLDL-triglyceride is present (15).
Moreover, results of the present study showed the lower level of TG in the hexagonal-treated group, which could be due to better control of glucose level and insulin sensitivity and maybe result of more insulin secretion from pancreatic B-cells.
According to the results of the present study, we concluded that the administration of hexagonal water can be useful for diabetic patients and made better hydration and metabolic condition by having insulin-like action with decreasing the glucose and TG levels in the STZ-induced diabetic rats. However, further researches are required to exactly detect the mechanism.