1. Background
Prostaglandins are produced by many tissues in the body; but in the present study, we were concerned primarily about the type produced by the uterine lining or endometrium. Uterine prostaglandins enter the blood stream and are eventually transported to the ovaries where they cause destruction or lysis of the corpus luteum (CL) and as a result, they cause a decrease in blood progestrone levels. Aspirin is one of the major drugs that inhibits the activity of prostaglandins. Prostaglandin inhibitor drugs inhibit the luteolysis process that leads to the prolongation of the CL activity in pregnant mice (1-6). Natural killer (NK) cells are one of the most important cell populations of the innate immune system with diverse effector functions. These cells are the main population of lymphocytes in the endometrium and especially in decidua. Decidual NK cells produce a number of cytokines and other secretory factors that help with trophoblast proliferation and differentiation and promote pregnancy success (7-9).
Considering the cyclic changes in the population of NK cells of uterus, it seems that their distributions and functions are under the control of ovarian hormones; estrogen and progesterone (9-11). Steroidal hormones cause major alterations in the endometrium and directly affect the uterus receptivity for accepting the blastocyst (4, 12). The differentiation of uterine NK (uNK) cells is regulated indirectly through estrogen and progesterone (7, 13). uNK cells are activated during days 5 - 7 of pregnancy, showing several alterations during this period such as change in superficial glycosylation of these cells, proliferation and simulation of IFNγ production, perforines, serines, estrases and other factors that are involved in angiogenesis (14).
Lee et al. stated that the immunological cells played an important role in the stabilization and retention of pregnancy (15). Immune cells show an important regulatory role in the uterus during early pregnancy and the mother has immune tolerance for the allo-antigenic tissue of the embryo (16). The embryo is considered to be semi-exotic to the mother, but the effect of ovarian hormones on the endometrium results in the differentiation of this tissue during the immunological response compared to other tissues; conversely, the embryo continues its growth (17).
Greenwood et al. and Guimond et al. reported that during pregnancy, uNK cells are activated and it seems that the secreted factors of these cells play an important regulatory role in the expression of vascular tissue genes as well as for the endometrial decidua (8, 18). Guimond et al. also investigated the role of NK cells during pregnancy and on the endometrial decidua (18). The role of NK cells in regulating the diferentiation of stroma cells and the response of related vessels during the implantation process has been demonstrated clearly (19-21). An increase in the amount of these cells results in the decrease of implantation and increases the abortion rate (10, 11, 22).
Based on the histological reports, low dosed of aspirin improve the percentage of implantation in females with thin endometrium who receive oocyst (23). There has been conflicting reports regarding the effect of low-dose aspirin on the hemodynamic condition of uterus (24-27). There has not been any comprehensive studies regarding the immunomodulatory role of aspirin on the NK cells of body tissues (28). In addition, there are conflicting reports regarding the effect of aspirin on the NK cells of other tissues. The studies investigated the effect of 660 mg of aspirin on the NK cells activity in patients with melanoma; the results showed that aspirin could suppress 80 - 100% of the NK cells (29).
However, another study using culture media showed that aspirin cannot suppress the NK cells activity stimulated by INFβ or spontaneus stimulation (30). These variations indicate that more research is needed to understand the effect of aspirin on the NK cells in different tissues (28). Based on the studies, low dosage of aspirin results in an alteration in the levels of ovarian steroidal hormones; however, the aspirin regulatory role in the NK cells in different tissues has not been investigated yet.
2. Objectives
Since pregnancy is one of the major factors in changing the amount of immune cells throught alterations in hormonal condition and regarding the fact that using low dosages of aspirin results in an improve in the implantation condition, this study was undertaken to investigate the effect of low dosage of aspirin on the amount of NK cells in the endometrium at the seventh day of pregnancy.
3. Materials and Methods
Adult female (6 - 8 weeks old) mice were cared and housed for two weeks in the laboratory for better adaptation. The female mice were placed beside the male mice and then, the mice with positive vaginal plug were separated using vaginal plug investigation method. The selected mice were divided accidentally into two study groups of control and experimental (using aspirin).
In the Experimental group, a low dosage of aspirin (7.5 mg/kg) was injected twice a day to the adult mice.
To determinate the amount of estrogen and progesterone, blood samples were obtained from the hearts of five pregnant mice selected from both groups on day seven of pregnancy. The concentrations of hormones in the sera were measured with enzyme-linked immunosorbent assay (ELISA) method.
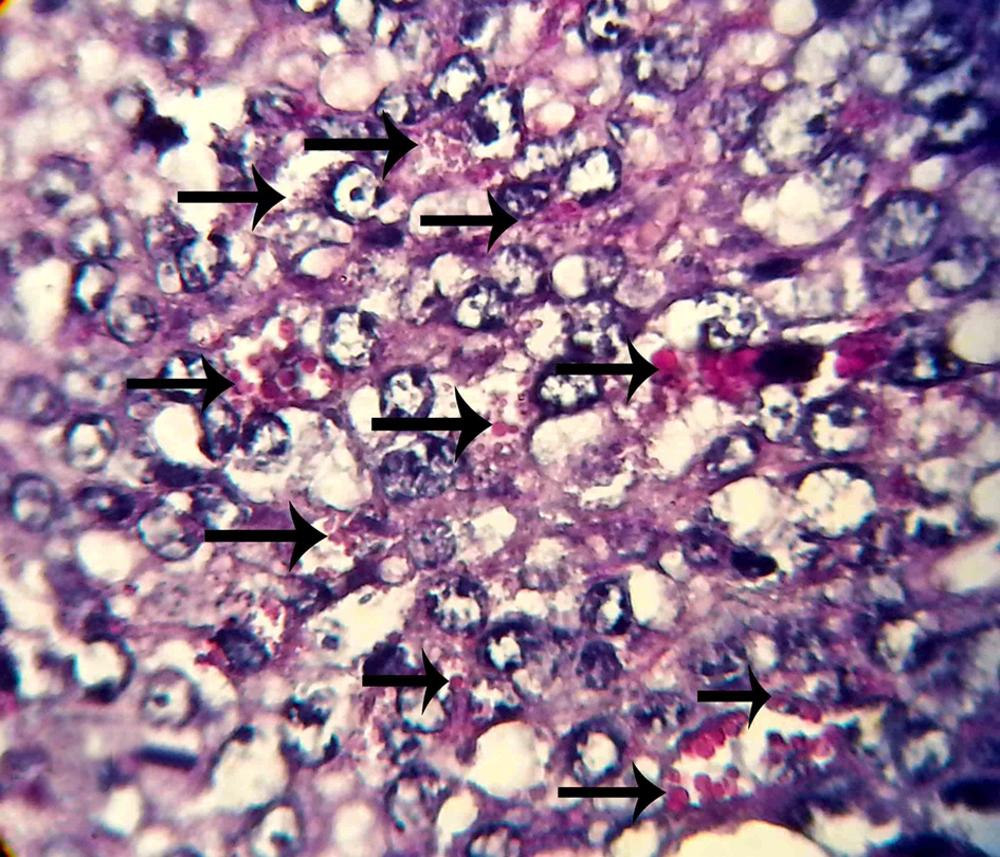
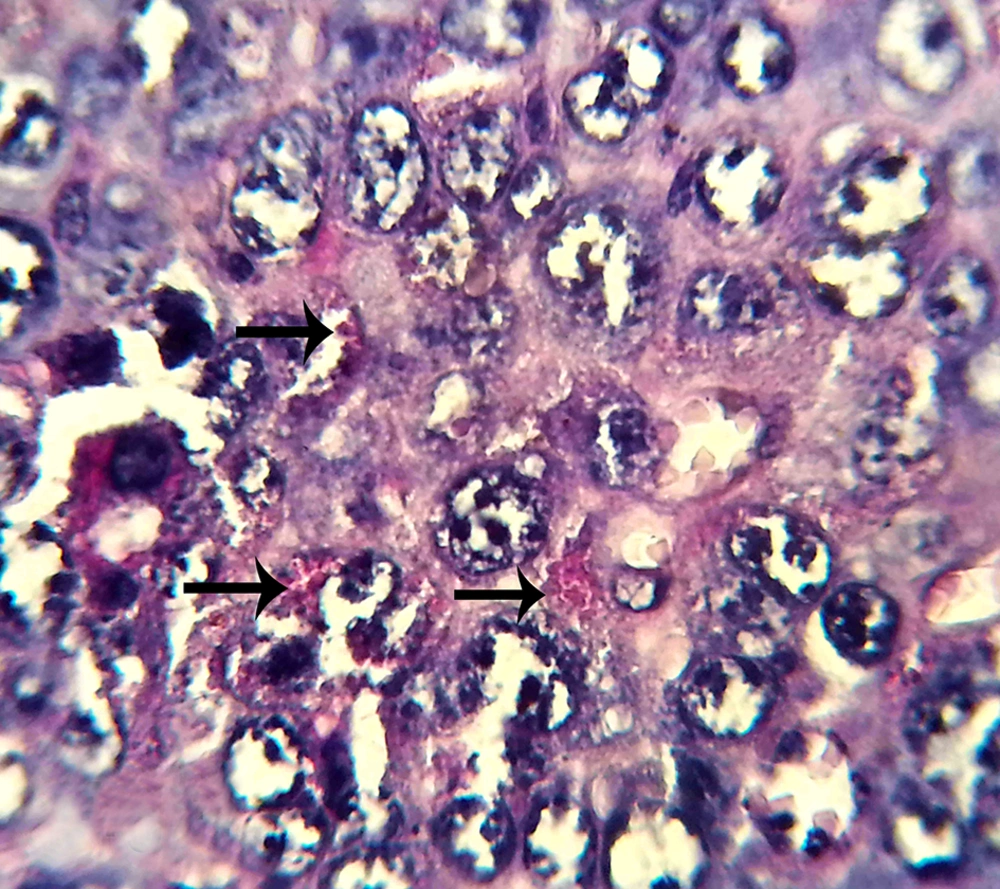
Five pregnant mice were sacrificed by cervical dislocation in each group in the seventh day of pregnancy. Their uterine horns were isolated from any fat and mesentery; then, the tissue samples were prepared from the uterine horns. Paraffin sections of 5 µm thickness were prepared (by serial section method). For NK cell counting, the sections were stained using periodic acid Schiff (PAS) method. From the prepared histological slides, one section was selected from each of the five sections and five microscopic fields in each section were analyzed. In the next step, the average of uterine stroma NK cells was calculated separately in each group. The results obtained from each group were compared using Mann-Whithny test. Statistical analysis was performed using statistical package for the social sciences (SPSS) and P values of less than 0.05 were considered statistically significant.
4. Results
The results of hormonal evaluation using Mann-Whithny test showed that there was a significant difference in the estrogen levels between the experimental and control groups (P < 0.05). Furthermore, the results of hormonal evaluation using the same test showed that there was a significant difference in the progesterone levels between the experimental and control groups (P < 0.05) (Table 1). The statistical evaluation of the NK cells showed a significant difference between the proportion of these cells in both groups (P < 0.05) (Table 2).
| Level of Hormone | Control Group | Experimental Group b |
|---|---|---|
| Estrogen, pg/mL | 69.79 ± 3.27 | 114.29 ± 6.74 |
| Progestrone, ng/mL | 38.37 ± 2.35 | 118.45 ± 6.2 |
aValues are presented as Mean ± SD.
bSignificant difference was seen with the control group (P < 0.05).
aValues are presented as Mean ± SD.
bAbbreviation: uNK: uterine natural killer.
cSignificant difference was seen with the control group (P < 0.05).
The micrographs indicated the presence of NK cells (positive PAS granules) in the uterine stroma of both groups (Figures 1 and 2).
5. Discussion
Aspirin is the inhibitor of prostaglandins. Prostaglandins affect the endometrium, resulting in the destruction of the CL and as a result, a decrease in blood progestrone levels (2, 3). Prostaglandin inhibitor drugs such as aspirin extend the duration of CL activity in pregnant mice due to the pause of luteolysis (4). The results of this study indicated that the administration of low-dose aspirin increased the level of estrogen compared to the control group, resulting in a significant statistical difference (P < 0.05); also, evaluating the results of progesterone determination showed a significant difference between the control and experimental groups (P < 0.05).
Al-Janabi showed that low dosage of aspirin resulted in the reinforcement of endometrium, size and quality of CL (31). Saki et al. demonstrated that using low-dose aspirin led to an increase in the number of CLs (32), as the results of the present study also confirmed the same findings.
Using low dosage of aspirin affects the ovaries, leads to an increase of estrogen and progesterone levels and alterations in the endometrium (33-35). Ovulation induction results in an increase in estrogen and progesterone levels and this phenomenon leads to a decrease in the number of NK cells (36-38).
The number of uNK cells in mice changes during sexual cycles and pregnancy. These cells can be found in the decidua during the implantation and are activated in day seven of pregnancy (39). Regarding the periodical changes of NK cells, it seems that they are under the control of the ovarian hormones (40, 41). NK cells play a fundamental role during the first stages of pregnancy, as the secretory factors of these cells are considered as the regulatory mediators in the expression of vascular tissue genes, decidualization reaction and survival of pregnancy; however, increase in the number of NK cells leads to an increase in abortion (8, 13, 15, 19, 42). The results of this study indicated that the treatment group using low-dose aspirin showed a significant statistical decrease in the number of uNK cells compared to the control group (P < 0.05).
In the present study, the administration of low dosage aspirin increased the level of estroidal hormones through affecting on the ovarian follicules. The results indicated that the injection of low-dose aspirin (7.5 mg/kg) caused significant differences in the levels of estrogen and progesterone. Seemingly, aspirin affected the ovaries and CL, leading to an increase in estrogen and progestrone levels. Finally, the endometrium underwent profound changes which were regulated by these hormones such as alterations in the number of NK cells.
It seems that the administration of low dosages of aspirin can lead to desirable alterations in endometrium and increase the pregnancy rate by affecting the proportion of NK cells within the uterus. Considering the important role of these cells in the establishment of a successful pregnancy, using a low dosage of aspirin (7.5 mg/kg) may affect the development of embryo and implantation.