1. Background
Different species of herbs have been used for centuries to prevent and control various diseases. Herbal extracts have been shown to be effective because they interact with specific chemical receptors within the body and can be considered as potent drugs in a pharmacodynamic sense (1). Moreover, interest in drugs derived from medicinal plants has markedly increased in the field of dentistry, and some species have been cited previously for the beneficial effects in the treatment of oral diseases (2, 3). One such species is Plantago australis Lam., a native plant found in southern Brazil, other Latin American countries, and the western part of the United States (4). This species is a perennial plant that belongs to the Plantaginaceae family and known as tancagem, trancagem, or tranchagem, depending on the geographical area within southern Brazil. Traditionally, P. australis has been used in folk medicine as an antibiotic, anti-inflammatory, and cicatrizing agent (4, 5). These biological activities are associated with a great number of active compounds, such as plantamajoside, baicalein, hispidulin, aucubin, ursolic acid, and oleanolic acid, each of which can be found in the leaves of the Plantago species (6-8). Nevertheless, there are still no reports on the cytotoxicity of the ethanol extracts of the P. australis species. This makes the current study of the possible effects of this plant species important to the findings of previous clinical tests.
The world health organization (WHO) has estimated that 80% of the world’s population uses medicinal plants in health care, and assistance has been provided to these countries in order to improve public health programs in this area (9-11). Brazil is considered to be one of top 10 consumers of herbal medicines, and 16 federative states in Brazil have introduced herbal medicines into the public health system (10, 11). Therefore, clinical studies are critical in potentially increasing the development of new and effective phythomedicines (2, 12) that can contribute to the expansion of the therapeutic options offered to users of the public health system in Brazil (11). However, few in vivo studies have been found to support the rational use of phytotherapeutic agents in dentistry (1, 12-15), and the value of routine oral pathology treatment needs to be emphasized in public health programs.
2. Objectives
This study investigated the cytotoxicity of the crude ethanolic extract of P. australis species in different concentrations through an in vitro assay.
3. Materials and Methods
3.1. Plant Material
Fresh whole specimens of P. australis (Figure 1) were collected in September of 2008 from the city of Caraa in southern Brazil. A voucher specimen of P. australis was deposited at the Federal university of Pelotas Herbarium (Capao do Leao, Brazil) under the number code PEL23.993. The plants were collected, rolled up in papers, and packed in cardboard pouches. Then, the plants were cleaned, and the leaves were separated from other aerial vegetable parts. Next, the leaves were dried in a stove with the air circulation set at 40°C for three days.
3.2. P. australis Extract
The leaves were harvested during the flowering phase, washed, and dried at 40 °C for 72 hours. They were then ground into powder using a pestle and mortar. Afterward, the powdered leaves were submitted for extraction with 70% v/v cereal alcohol (hydro-alcoholic extract). Initially, 200 g of this dry vegetable biomass of P. australis were added to 850 mL of ethanol (85% of the volume of 1000 mL of ethanol) and periodically agitated during the course of 48 hours. The ethanol and P. australis mixture was stored in a sterile amber glass vial protected from light exposure and maintained at room temperature. After 48 hours, the mixture was filtrated through a paper filter, and the liquid was stored in another sterile amber glass vial. The vegetable residue was then re-extracted with 150 mL of ethanol corresponding to 15% of the volume of 1000 mL of ethanol for an additional 48 hours. After the filtration process, the P. australis crude extract was stored in a sterile amber glass vial in a refrigerator (4°C) until it was ready to be used. The protocol for obtaining the tinctures of the medicinal plants was adapted from both the Brazilian and the British pharmacopoeia guidelines (16, 17).
3.3. In vitro Cytotoxicity
3.3.1. Cell Culture
The immortalized cell line 3T3 fibroblasts were cultured in T25 flasks according to the recommendations from the ATCC (American type culture collection, USA) in a mixture of Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) containing 10% fetal bovine serum (FBS), 2% L-Glutamine, 100 U mL-1 penicillin, and 100 mg mL-1 streptomycin. After adequate confluence was obtained, there was a viable cell count with trypan blue, and the cells were plated at a density of 2 × 104 cells/well in 96-well culture plates (Corning-Costar) for subsequent cytotoxicity assay.
3.3.2. The Dimethyl Thiazolyl Diphenyl Tetrazolium Salt (MTT) Reduction Method
The objectives of this method consisted of the absorption of bromide salt MTT {[3 - (4, 5-dimethylthiazol-2-yl) -2, 5-diphenyltetrazolium bromide]} (Sigma) by the cells, followed by a reduction within the mitochondria to a product called formazan. This product that accumulated inside the cell was extracted through the addition of a suitable solvent (18).
After 24 h of plating and incubating the cells at 37°C in an incubator at 5% CO2, serial dilutions of crude ethanolic extract of P. australis at 70% were incubated in contact with the cells for 24 hours. In this study, the following serial dilutions of DMEM supplemented with 10% of FBS were used: 10x, 100x, 1000x, 10000x, and 100000x. After the incubation period, the extract was removed from contact with the cells, and the plate was washed with phosphate-buffered saline (PBS), each well with 200 µL. Then, a solution of MTT dye was mixed with the medium at a concentration of 5 mg/mL and incubated in the dark for a further 4 hours in contact with the cells. Then, the medium was carefully removed, and 200 µL of dimethylsulfoxide (DMSO, Sigma) was added into each well to solubilize the formazan crystals. The plates were agitated for five minutes, and the absorbance for each sample was measured with an ELISA reader (enzyme-linked immunosorbent assay) at 560 nm. The control group was determined using DMEM with no addition of extract in contact with the cells, whereas the white controls were determined using a DMEM medium with no presence of cells. There were eight replicates for each group, and the entire experiment was repeated once.
3.4. Statistical Analysis
The cell viability data (absorbance) were statistically analyzed by one-way ANOVA followed by a multiple-comparison Tukey’s post hoc test (P < 0.05) for each dilution. A value of P < 0.05 was considered to be statistically significant.
4. Results
4.1. Cytotoxicity Assay (MTT Reduction) in vitro
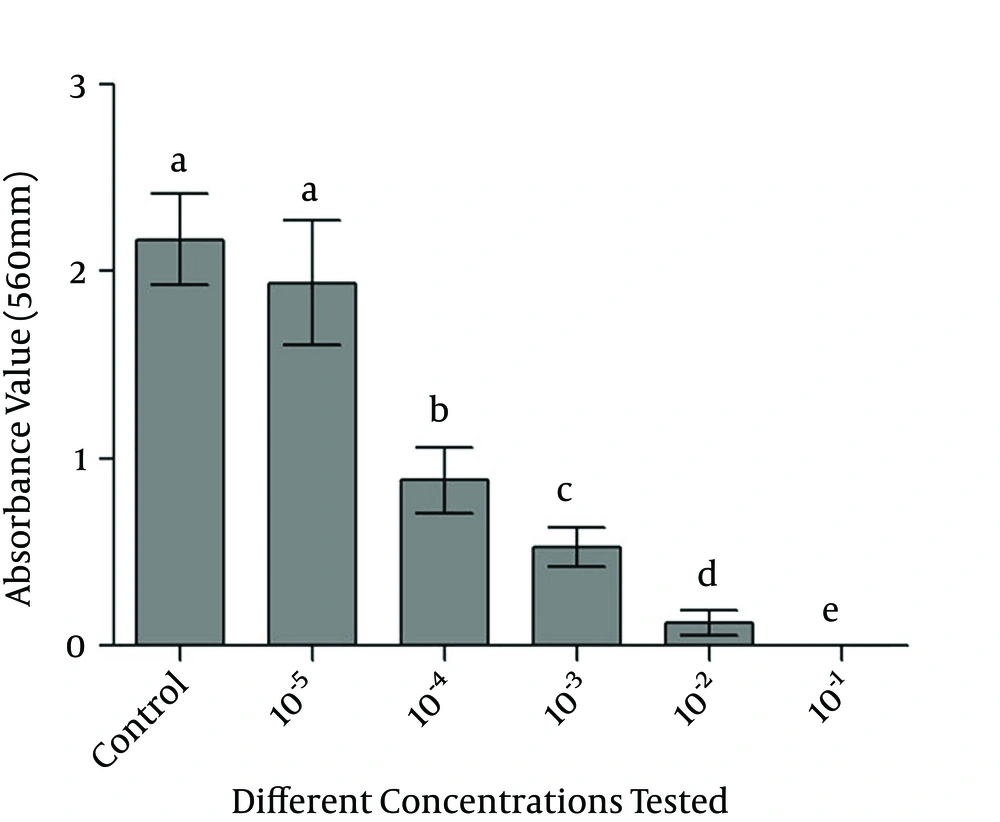
The results for the MTT reduction assay in salt formazan crystals by mitochondrial activity after contact with the monolayer cell at different dilutions of the crude extract of P. australis are shown in Figure 2. In this experiment, the more concentrated the crude ethanol extract of P. australis, the stronger the observed cytotoxicity effect. Moreover, only the group most diluted had behaved similarly to the control group (cells with no addition of the extract).
5. Discussion
Plantago major, another species belonging to Plantaginaceae family was the second plant considered to be most important to the southern population in an ethnomedicinal survey of professionals and patients in the basic care units of the Brazilian unified health system (10, 11). Moreover, the Brazilian ministry of health published a list of 71 medicinal plants that are considered potentially useful for the production of phytomedicines and other compounds (11, 19), and Plantago major is included in this list, showing the popularity of its use and the potential of the Plantago genus plant species to be used as phytomedicine (11, 20).
Cytotoxicity assays are considered to be a useful tool for the initial screening of the toxic effects of medicines (21, 22). The control of the physicochemical and physiological environment of the experiment, the reduction of animal experiments, and reproducibility are some of the advantages of cytotoxicity assays (23). However, one can consider that the main limitation of these tests is the difficulty in reproducing in vivo conditions (21). Accordingly, the results of the cytotoxicity assay of this study should be analyzed with caution because there are no previous trials in the literature that assess the cytotoxicity of the ethanol extracts of the P. australis species, and also because of the presence of a single monolayer of cells exposed to the ethanol extract at a concentration of 70% alcohol, which may have been responsible for the observed high cytotoxicity at less diluted concentrations. Thus, it is clear that further studies with other methodologies should be conducted to complement the results of the present study. For instance, tests for the assessment of genetic damage to cells exposed to this extract may complement the results.
5.1. Conclusions
It can be concluded that the crude extract of P. australis was cytotoxic in the least diluted concentrations against 3T3 fibroblasts.