1. Background
Cell transplantation and testicular grafting of small pieces of testicular tissue from a living donor to a receiver are two new tools that may be used in the future for treatment of infertile men. This technique allowed the study and manipulation of various aspects of spermatogenesis (1-5). Transplantation of fresh testicular cells or tissues is not always possible. Thus, the ability to preserve testicular tissue cells appears to be necessary for future use. Testes contain spermatogonial stem cells that are capable of proliferation and differentiation. Effective preservation of testes structure may allow them to resume normal proliferation (1-5). Cryopreservation of animal and human testicular cells has been done successfully, but too little attention has been paid to structure of testicular tissue during cryopreservation. Testicular tissue cryopreservation can be performed in cases that sperm cryopreservation is not possible. Preservation of testicular tissue used in children rescued from cancers such as neuroblastoma, lymphoma, Hodgkin disease, osteoarthritis sarcoma, Ewing’s sarcoma, rhabdomyosarcoma, Wilms’ tumor and non-Hodgkin lymphoma (1-5).
Spermatogenesis in adolescence may be impaired following radiotherapy and chemotherapy. There are different vitrification protocols for cryopreservation of immature testicular tissue but fine structure and cell viability of testes have not been preserved. Several studies examined pieces of frozen testes or testicular cell suspension using glycerol, ethylene glycol, dimethyl sulfoxide, and propanediol, but none were able to maintain high performance quality of spermatogonial stem cells (6). Genetic defects in the embryo vitrification have also created concerns similar to what is happening in slow freezing (7). Recently, supplementation of freeze-thaw media with antioxidants such as vitamin E, ascorbic acid, and selenium are considered. Another studies showed that the number of apoptotic cells increases in the vitrification solution on the first day of the thawing (8, 9). Many studies have been done to reduce vitrification cryoinjury. Recently, researchers have suggested that supplementation of vitrification and thawing media with antioxidants may reduce cryoinjury (10-13). In another study, it was reported that supplementation vitrification and thawing solution of Dimethyl sulfoxide (DMSO) 15% and ethylene glycol (15%) with melatonin did not protect testicular tissue from injury (14). Melatonin is small biological molecule that is secreted in the pineal gland and other organs e.g., retina and testis (15-17). Effects of melatonin are studied in many regulatory functions of cells such as immune response, cell signaling, protecting fatty acids from oxidation and nuclear DNA from damage, controlling tumor growth and inhibiting cell proliferation, oncostatic action, anti-apoptotic effect on many normal cells, enhancing apoptosis in the tumor cells and significant anti-aging properties (15-29). The raised question was whether adding melatonin on other cryoprotectant media, based on glycerol, DMSO, ethylene glycol or mixture of them, can reduce cryoinjury.
2. Objectives
The study aimed to investigate the antioxidant effects of melatonin on histological structure of seminiferous tubules that are vitrified-thawed with different cryoprotectant media.
3. Materials and Methods
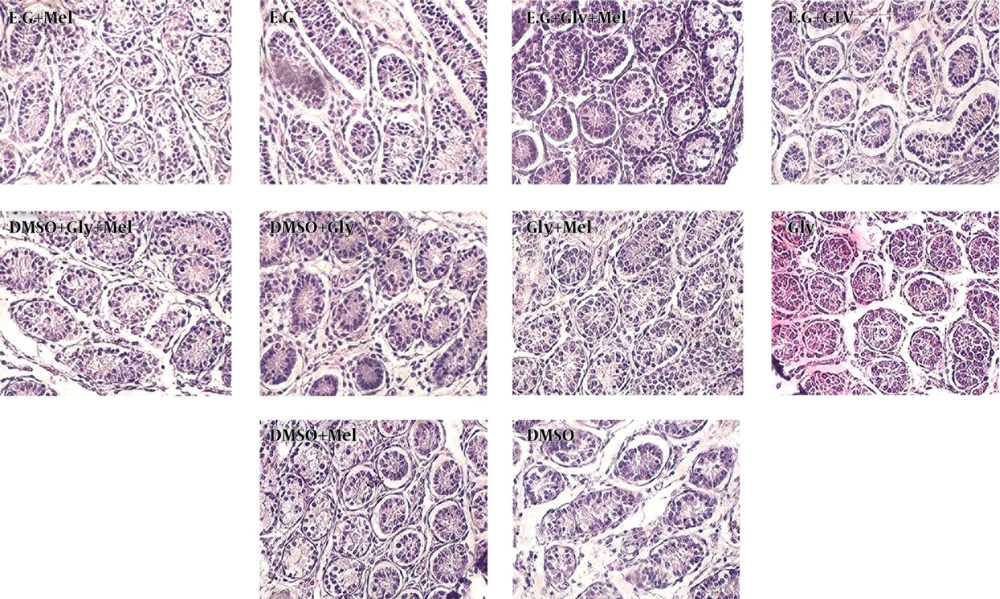
All experiments were performed in accordance with the principles of laboratory animal care. A total of 50, 6-day-old BALB/c male mice pups were obtained from physiology research center. Mice were euthanized by excessive doses of ketamine HCl (80 mg/kg) and xylazine (10 mg/kg) (Pharmacia and Upjohn, Erlangen, Germany) in accordance with the protocols approved by the Ahvaz Jundishapur University Medical Science Animal Care and Use Committee. Male mice were randomly assigned to one of ten experimental groups. Testes of 6-day-old BALB/c male mice pups were extracted and vitrified-thawed according to Table 1. Samples after thawing were fixed with bouin’s fixative. After 24 hours fixation, samples processed and stained with hematoxylin-eosin staining. Results of treated group with melatonin and control group (untreated group) were compared with Mean-Whitney U test and all groups were compared with Kruskal-Wallis H Test by SPSS 16.
| Sucrose, 0.5 M | Ethylene Glycol | DMSO | Glycerol | FBS 20% | Melatonin 100 µg/mL | |
|---|---|---|---|---|---|---|
| Group 1 | + | 40 | - | - | + | + |
| Group 2 | + | 40 | - | - | + | - |
| Group 3 | + | 7.5 | - | 7 | + | + |
| Group 4 | + | 7.5 | - | 7 | + | - |
| Group 5 | + | - | 7.5 | 7 | + | + |
| Group 6 | + | - | 7.5 | 7 | + | - |
| Group 7 | + | - | - | 15 | + | + |
| Group 8 | + | - | - | 15 | + | - |
| Group 9 | + | - | 15 | - | + | + |
| Group 10 | + | - | 15 | - | + | - |
a Abbreviations: DMSO, Dimethyl sulfoxide; and FBS, Fetal Bovine Serum.
b Data are presented as (%).
4. Results
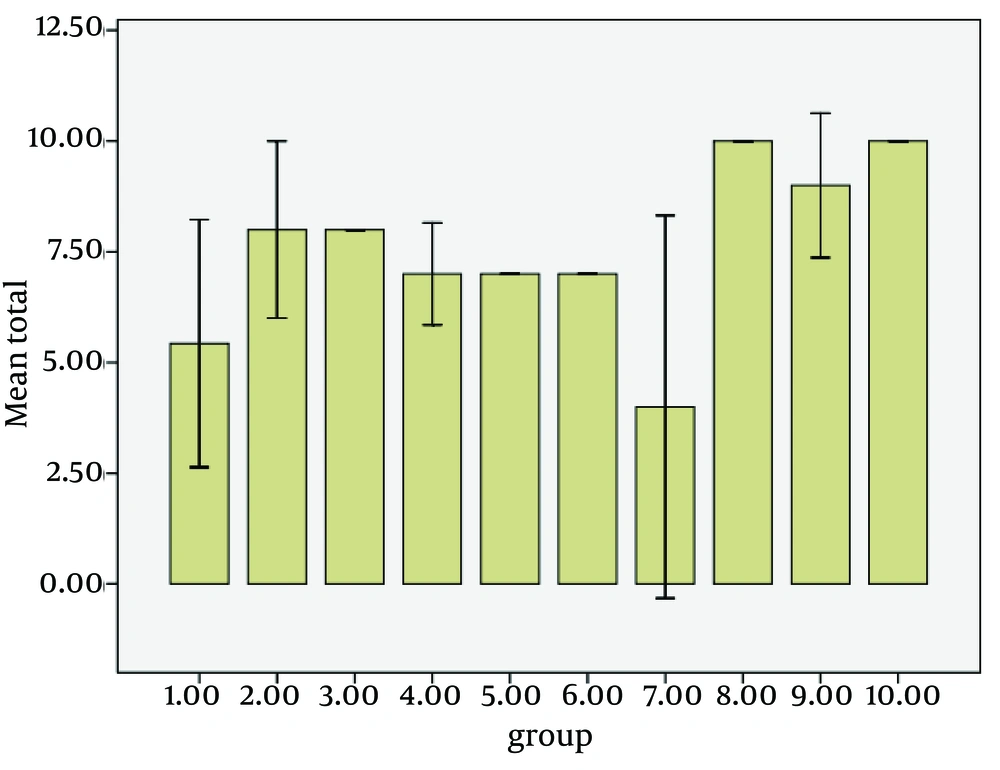
Tissue sections after vitrification using hematoxylin-eosin staining were studied and analyzed according to Milazzo et al. method (30). The blind evaluation of tissue sections was done by specialists. Statistical analysis of comparing seminiferous epithelium glycerol based vitrification media supplemented with melatonin (group 7) to the control group (group 8) was significant (P = 0.004) (Figures 1 and 2). Statistical analysis of comparing seminiferous epithelium ethylene glycol based vitrification media supplemented with melatonin (group 1) with control group (group 2) was not significant (P = 0.226). Statistical analysis of comparing seminiferous epithelium ethylene glycol and glycerol based vitrification media supplemented with melatonin (group 3) to the control group (group 2) was not significant (P = 0.529). Statistical analysis of comparing seminiferous epithelium DMSO and glycerol based vitrification media supplemented with melatonin (group 5) to the control group (group 6) was not significant (P = 0.529). Statistical analysis of comparing seminiferous epithelium DMSO based vitrification media supplemented with melatonin (group 9) to the control group (group 10) was not significant (P = 0.529). All statistical analyses were done according to Milazzo et al. method (30).
5. Discussion
Oxidative stress is a critical factor in the death of animal tissues during the vitrification process. Spermatogonial stem cells and testis tissues are exposed to multiple stresses during the vitrification process (31, 32). Choosing a cryoprotectant approved by all authors for freezing cells had not been realized yet; however, many researchers have recently done extensive researches about choosing best cryoprotectant for vitrification. Vitrification processes differ from species to species due to the nature of their tissues and thus require optimization for different animal tissues. Melatonin is anti-apoptotic and antioxidant, which is secreted from the pineal gland. In a previous study, it was demonstrated that supplementation of vitrified-thawed media, DMSO and ethylene glycol plus sucrose, with melatonin did not protect testicular tissue from injury (14). DMSO, which is used during cryopreservation in routine laboratory tasks, has a lot of toxic effects. Researchers recently proposed that DMSO has antioxidant activity (33). Researchers recently showed that supplementation of vitrified-thawed media with ethylene glycol or dimethyl sulfoxide or a mixture of ethylene glycol and dimethyl sulfoxide causes fewer toxic effects on the cryopreserved mesenchymal stem cells (34). In this study, supplementation of glycerol vitrification media with melatonin showed fewer toxic effects on the cryopreserved immature testicular tissue. Uchendu et al. (35) recently showed that supplementation of cryopreservation media, DMSO based, with melatonin enhances the recovery of cryopreserved shoot tips of American elm.
Recently Succu et al. (36) has shown that supplementation of post warming media with melatonin enhances efficiency of vitrified ovine embryos. They showed that supplementation post warming media with high concentration of melatonin (10-3) had toxic effect on vitrified ovine embryos but supplementation post warming media with low concentration (10-9) of melatonin may have beneficial effects on development of vitrified ovine embryos (36). In this study, supplementation of vitrification media, DMSO based, with melatonin did not protect testicular tissue from injury.
Taken together, it can be said that supplementation of vitrification media, glycerol based, with melatonin had better effect on reducing testicular tissue injury while using melatonin in other environments of freezing-thawing, e.g. DMSO based, ethylene glycol based, is less effective in reducing the damage caused vitrification. Supplementation of glycerol based vitrification media with melatonin reduces testicular tissue injury in the vitrification process.