1. Background
Anxiety disorders are highly widespread, with a growing global occurrence. The current treatment includes primarily benzodiazepines and selective serotonin reuptake inhibitors (SSRIs), two classes of drugs with numerous adverse effects (1, 2). Benzodiazepines are related to ataxia, sedation, skeletal muscle relaxation, amnesia and interplays with ethanol and barbiturates; while, SSRIs show slow beginning of the anxiolytic action (1, 3). Therefore, there is a need for strong anxiolytic compounds with minor adverse effects and instant start of action (4).
Flavonoids chemically are phenylbenzopyrones, generally conjugated with sugars and there are in all vascular plants. Numerous natural properties have been attributed to flavonoids. The flavonoids effects on the central nervous system have been measured only in the recent 10 years. Especially, studies performed by Medina confirmed the ability of a number of flavonoids to attach the central type benzodiazepine (BZD) receptors (5).
Ellagic acid (EA) is a dimeric derived from gallic acid. It is a naturally-synthesized phenolic composition present in fruits and nuts for example raspberries, strawberries, pomegranates, cranberries, walnuts, pecans and other plant foods (6). It displays anti-mutagenic (7, 8), antioxidant (9) and anti-inflammatory (10, 11) activities. In the nervous system, EA as well acts a significant function as an anti-carcinogen and antioxidant (12). EA could reduce reactive oxygen species (ROS) creation in astrocytes and dissuade astrocytic cell death resulted from Cd2+ exposure (13).
2. Objectives
The purpose of the present study was to investigate the effect of ellagic acid on motor activity and anxiety in open field test and elevated plus maze model of anxiety. To gain more information on the mechanism of action of ellagic acid, diazepam used as standard anxiolytic drug with different doses and flumazenil used as an antagonist of GABA-A Receptor.
3. Materials and Methods
3.1. Animals
Laboratory animals (male mice) weighing approximately 30 - 20 grams were obtained from Ahvaz Jundishapur University Animal Center. Animals kept under conditions of 12 hours light and 12 hours darkness. Male mice were randomly assigned to groups based on a pilot study with an adequate number. Ellagic acid was administered intraperitoneally half an hour before the test. Diazepam administered intraperitoneally (i.p.) at a dose of 5 mg/kg and 1 mg/kg (14) half an hour before the test. Flumazenil (3 mg/kg) was administered intraperitoneally (15) fifteen minutes prior to administration of ellagic acid. Ellagic acid administered intraperitoneally and half an hour later elevated plus maze and locomotor activity tests were performed.
3.2. Grouping of Samples
Total number of animals used in this study was 192 in 24 groups (8 per each group). The animals were divided into two groups of 12. In the first subgroup, elevated plus maze and in the second subgroup open field test were performed. Study groups are as follows: 1) Control Group received DMSO 5 mL/kg (single dose) 2) Group receiving 3 mg/kg (single dose), ellagic acid 3) group receiving 10 mg / kg (single dose), ellagic acid, 4) group receiving 30 mg/kg (single dose), ellagic acid, 5) group receiving 100 mg/kg (single dose), ellagic acid, 6) ineffective dose of ellagic acid with diazepam 1 mg/kg, 7) groups receiving diazepam 1 mg/kg (single dose), 8) groups receiving diazepam 5 mg/kg (single dose), 9) groups receiving ellagic acid 3 mg/kg with flumazenil, 10) groups receiving diazepam 5 mg/kg (single dose) with the dose of 3 mg/kg flumazenil, 11) Control group received DMSO (10 days, 5 mL/kg/day), 12) group receiving an effective dose of ellagic acid (daily for 10 days).
3.3. EPM Test
The method recommended by Handley and Mithani (1984) for measurement of exploratory behavior, was used with some modifications (16). EPM was constructed from black plexiglas. The equipment comprised of two opposed open arms (35 × 5 cm) with no side walls and two closed arms (35 × 5 × 20 cm) with side walls, extending from a central quadrangle (5 × 5 cm). In the current study, the guard lips were not used on the open arms. To make easy the account of exploratory action, open arms were separated by the lines into three equivalent divisions. The maze was as high as 40 cm from the floor, and located in a light room (~750 lux). The behavior of animals was recorded by a digital camera, which was then evaluated by a person not concerned in executing the experiment. Throughout a 5-min watching session, the following events were taken by an observer: 1) count of head-dipping; 2) count of line crossings on the open part; 3) time spend in travel over the open arms of plus-maze; 4) count of closed and open arm entries; 5) proportion between open and total arm entries. At the start of examination, animals were located on the center of plus-maze, facing in the direction of an open arm. An arm entrance was calculated only when all four limbs of the rat were inside a specified arm. The plus-maze was cleansed between tests using 5% alcohol solution (17).
3.4. Open-Field Test
To perceive any relation between immobility in experiments and alterations in motor activity, the movement of animals treated with Ellagic acid was evaluated in the open-field apparatus. The open-field device was made of acrylic (transparent walls and black floor, 30 × 30 × 15 cm), partitioned into nine quadrangles of equivalent areas. The open-field was used to assess the investigative movement of animals (18). The mice were positioned alone into the middle of the field and allowed to travel around it freely. Ambulation (the count of squares traversed with all four paws) and total number of grooming and rearing and sniffing actions were viewed for five minutes. The walls and floor surfaces were thoroughly cleaned with 5% ethanol between the tests (19).
3.5. Statistical Analysis
Data are reported as mean ± SEM. Result was analyzed by SPSS software (SPSS Statistics Ver. 21, IBM, USA). One-way ANOVA test was used for comparisons between different groups. Post Hoc Tukey’s test was used to assess differences between the groups. P < 0.05 was considered as significant.
4. Results
4.1. Elevated Plus Maze Test
4.1.1. Single-Dose Administration of Ellagic Acid in Doses of 3, 10, 30, 100 mg/kg and Diazepam in Doses of 1, 3, 5 mg/kg
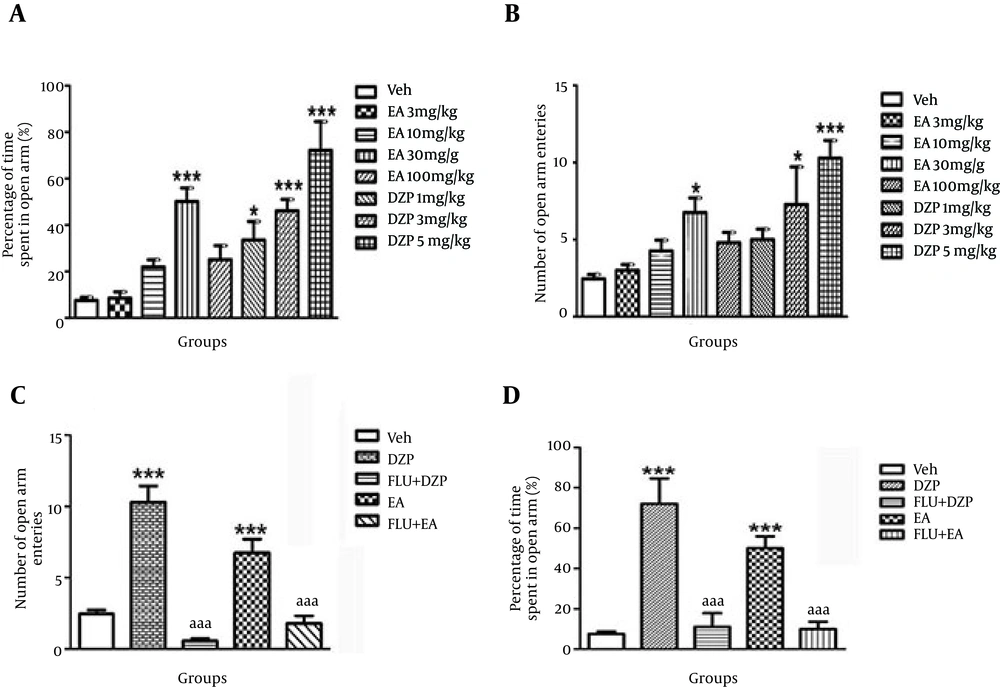
The results showed that administration of ellagic acid at doses of 3 and 10 mg/kg had no effect on the time spent and the number of open arm entries (P > 0.05) (Figure 1A and B). Ellagic acid at dose of 30 mg/kg enhanced the number of open arm entries and the time spent in open arm significantly compared with the control group (P < 0.05) (Figure 1A and B). In the group receiving 100 mg/kg ellagic acid, none of the parameters showed significant changes compared with the control group.
A) Percentage of time spent in EPM open arm in different groups (n = 8): control (Veh), received a single dose of EA (3, 10, 30 and 100 mg/kg, i.p.), DZP (1, 3 and 5 mg/kg, i.p.). *P < 0.05, ***P < 0.001, vs. control. B) Number of entries in EPM open arm in different groups (n = 8): control (Veh), received a single dose of EA (3, 10, 30 and 100 mg/kg, i.p.), DZP (1, 3 and 5 mg/kg, i.p.). *P < 0.05, ***P < 0.001, vs. control. C) Number of open arm entries in the EPM test in different groups (n = 8): Control (Veh), EA (30 mg/kg., i.p.), Diazepam (5 mg/kg, i.p.) and Flumazenil (3 mg/kg, i.p.). ***P < 0.001, vs. control and aaaP<0.001, vs. DZP. D) Percentage of time in open arm latency in different groups (n = 8). Control (Veh), EA (30 mg/kg., i.p.), Diazepam (5 mg/kg, i.p) and Flumazenil (3 mg/kg, i.p.). ***P < 0.001, vs. control and aaaP < 0.001, vs. DZP Abbreviations: DZP = diazepam, EA = ellagic acid, FLU= flumazenil, Veh = vehicle. Data in all sections, A - D, reported as mean ± SEM and analyzed using one-way ANOVA test followed by post Hoc Tukey’s test.
Administration of Diazepam at doses of 1, 3 and 5 increased the count of open arm entries and percentage of time spent in open arm significantly compared to the control group.
4.1.2. Co-Administration of Diazepam (5 mg/kg) with Flumazenil (3 mg/kg) and Co-Administration of Effective Dose of Ellagic Acid (30 mg/kg) With Flumazenil (3 mg/kg).
In the group receiving diazepam + ellagic acid, flumazenil (3 mg/kg) (GABA A-benzodiazepine receptor antagonist) first injected i.p., and then 15 minutes later, diazepam (5 ml/kg) injected i.p.
Single-dose administration of diazepam in comparison with controls significantly increased the number of entries in open arm (P < 0.001). Percentage of spent in open arm increased significantly (P < 0.001) (Figure 1C and D and Table 1).
| veh | EA30 mg | FLU + DZP | FLU + EA | DZP5 | |
|---|---|---|---|---|---|
| Open entries | 2.462 ± 0.2683 | 6.75 ± 0.9545 b | 0.555 ± 0.17 c | 1.8 ± 0.53 d | 10.3 ± 1.14 b |
| Ppen time % | 7.563 ± 1.277 | 50.1 ± 5.834 b | 11.1 ± 6.80 c | 10 ± 3.584 d | 72.1 ±12.4 b |
| Close entries | 11.58 ± 1.145 | 6.125 ± 1.083 e | 12.56 ± 3.024 | 11.25 ± 3.7 f | 3.25 ±0.94 |
| Time close% | 92.58 ± 7.866 | 49.85 ± 2.933 b | 79.13 c ± 7.44 | 90.29 ± 3.2 d | 28.55 ± 6.9 b |
| Total arm entries | 14.042 ± 1.4133 | 12.875 ± 2.0375 | 13.11 ± 3.2 | 13.05 ± 4.3 d | 13.54 ± 2.0892 |
aGroups: Control (Veh), Diazepam (5 mg/kg, I.P.), Ellagic acid (30 mg/kg, I.P.), Diazepam + flumazenil (5 mg/kg, I.P. and 3 mg/kg, I.P., respectively), Flumazenil + Ellagic acid (3 mg/kg, I.P. and 30 mg/kg, I.P., respectively).
bP < 0.001 vs. control.
cP < 0.001 vs. Diazepam (5mg/kg).
dP < 0.001 vs. EA (30 mg/kg).
eP < 0.05 vs. control.
fP < 0.05 vs. EA (30 mg/kg).
Administration of flumazenil concomitant with effective dose of diazepam significantly decreased the number of open arm entries (P < 0.001) and percentage of time in open arm compared with diazepam alone (P < 0.001) (Figure 1C and D and Table 1).
4.1.3. Co-Administration of Ineffective Dose of Ellagic Acid (10 mg/kg) With Ineffective Dose of Diazepam (1 mg/kg) on EPM Test Parameters
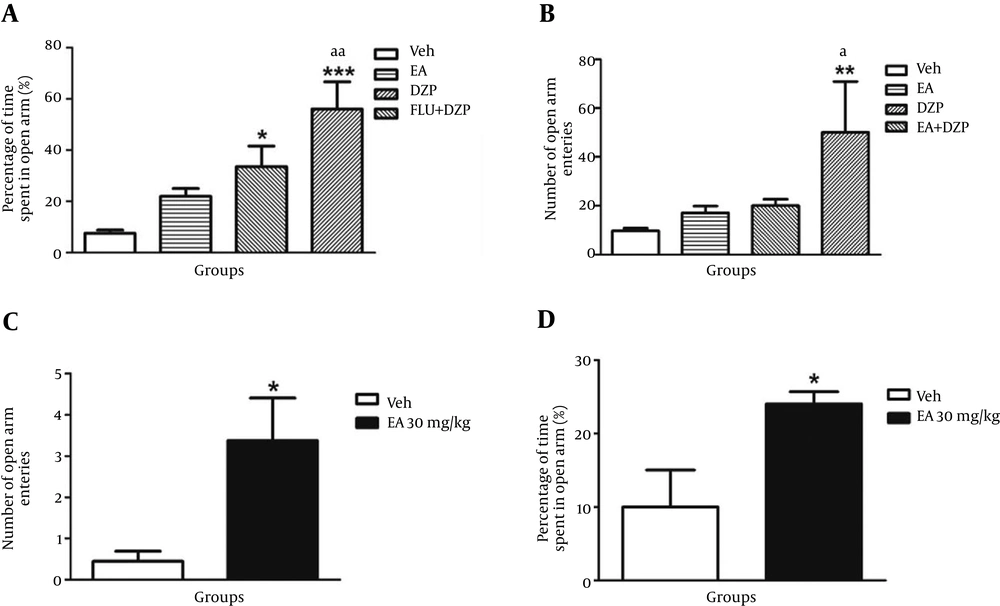
In this group, at first ineffective dose of diazepam (1 mg/kg) was injected i.p. and then 15 minutes later Ellagic acid (10 mg/kg) was administered i.p. Administration of ellagic acid (10 mg/kg) solely had no significant effect on the number of entries and time spent in open arm. However, diazepam (1 mg/kg) had no effects on these parameters in EPM. Co-administration of ellagic acid (10 mg/kg) and diazepam (1 mg/kg) increased count of entries and percent of time spent in open arm significantly (P < 0.01) compared with control group as well as with ineffective dose of ellagic acid and diazepam (Figure 2A and B and Table 2).
A) Percentage of time spent in open arm between different groups (n = 8): control (Veh), EA (10 mg/kg), Diazepam (1 mg) and EA + Diazepam, 10 mg/kg and 1 mg/kg, respectively). * P < 0.05, ***P < 0.001, vs. control and aaP < 0.01, vs. EA (30 mg/kg). B) Number of entrance between different groups (n = 8): control (Veh), EA (10 mg/kg), Diazepam (1 mg) and EA + Diazepam, 10 mg/kg and 1 mg/kg, respectively). **P < 0.01, vs. control and aaP < 0.01, vs. EA (30 mg/kg). C) Number of entries in open arm in EPM between control and chronic administration of EA (10 mg/kg/day. i.p.) (n = 8). *P < 0.05. D) Percentage of time spent in open arm in EPM between control and chronic administration of EA (10 mg/kg/day. i.p.) (n = 8). *P < 0.05. Abbreviations: DZP = diazepam, EA = ellagic acid, Veh= vehicle. Data in all sections, A - D, reported as mean ± SEM and analyzed using one-way ANOVA test followed by post Hoc Tukey’s test.
| Veh | EA10 mg | DZP1 mg | DZP1+ EA10 | |
|---|---|---|---|---|
| Open entries | 2.462 ± 0.2683 | 4.273 ± 0.7018 | 5 ± 0.6853 | 12.5 ± 5.23b,c |
| Open time % | 7.563 ± 1.277 | 21.99 ± 3.036 | 33.54 ± 8d | 56 ± 10.6e,f |
| Close entries | 11.58 ± 1.145 | 8.25 ± 0.836 | 6.077 ± 1.643d | 1.6 ± 0.68e,f |
| Time close% | 92.58 ± 7.866 | 77.5 ± 7.237 | 66.02 ± 9.059 | 44.37 ± 8.93b |
| Total arm entries | 14.042 ± 1.4133 | 12.523 ± 1.5378 | 11.077 ± 2.3283 | 14.125 ± 5.9087 |
aGroups: Control, Diazepam (1 mg/kg, I.P), Ellagic acid (10 mg/kg, I.P), Diazepam + EA (1 mg/kg, I.P and 5 mg/kg, I.P, respectively). Data reported as mean ± SEM and analyzed using one-way ANOVA followed by post Hoc Tukey’s test. (DZP = diazepam, EA = Ellagic acid) (n = 8).
bP < 0.01 vs. control.
cP < 0.05, vs. EA (10 mg/kg).
dP < 0.05 vs. control
eP <0.001 vs. control.
fP < 0.01, vs. EA (10 mg/kg).
4.1.4. Chronic (10 Days) Administration of Effective Dose of Ellagic Acid (30 mg/kg, i.p.) on EPM Test
EA (30 mg/kg/day) was administered for 10 day. The results showed that the number of entries in open arm and percentage of time spent in open arm significantly increased compared with the control group (P < 0.05) (Figure 2 C and D) (Table 3).
aGroups: control (veh) and chronic administration of EA (10 mg/kg/day. i.p.) (n = 8).
bData reported as mean ± SEM and analyzed using one-way ANOVA followed by post Hoc Tukey’s test. (EA = ellagic acid, Veh= vehicle).
cP < 0.05.
dP < 0.01.
4.2. Motor Activity in Open Field Test
4.2.1. Effect of Different Doses of Ellagic Acid (3, 10, 30 and 100 mg/kg) and Diazepam (1, 3 and 5 mg/kg) on Motor Activity in Open Field Test
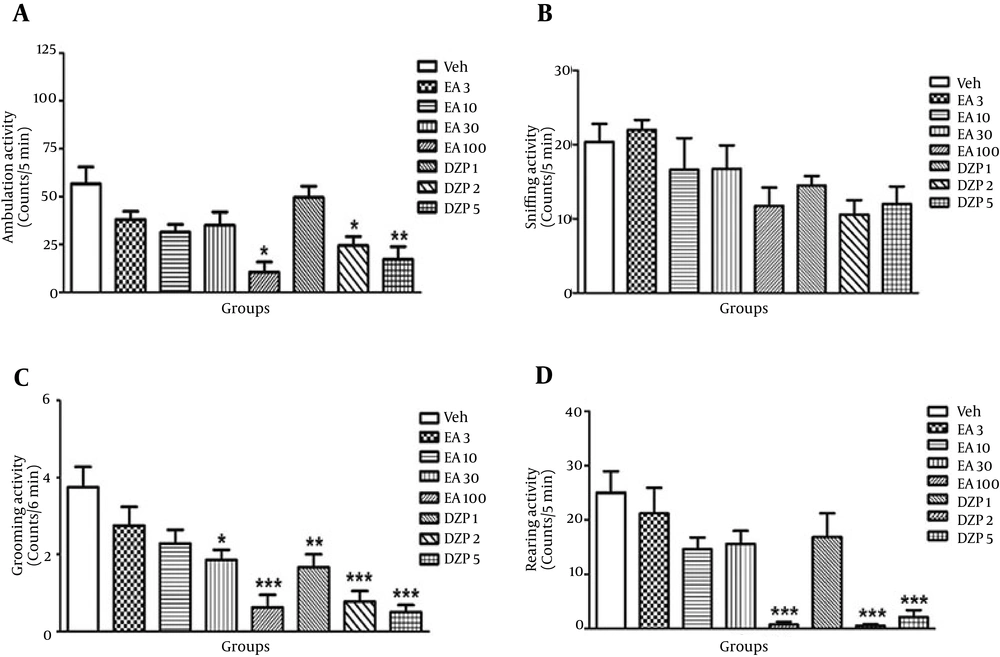
In all groups, at first EA (3, 10, 30 and 100 mg/kg, i.p., single dose) was injected, and after 30 minutes animals were placed in open field apparatus and motor activity parameters were recorded. EA at doses of 3 and 10 mg/kg had no effect on motor activity in open field apparatus. EA at dose of 30 mg/kg just decreased sniffing compared with the control group (P < 0.05), but other parameters such as ambulation, rearing and grooming did not alter. EA at dose of 100 mg/kg decreased ambulation, grooming and rearing significantly compared with the control group, but sniffing did not alter compared with the control group (Figure 3 A-D).
A) Comparison of ambulation activity between different groups in the open field test (n = 8). *P < 0.05, **P < 0.05, vs. control. B) Comparison of sniffing activity between different groups in the open field test (n = 8). C) Comparison of grooming activity between different groups in the open field test (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001, vs. control. D) Comparison of rearing activity between different groups in the open field test (n = 8). ***P < 0.001, vs. control. Abbreviations: DZP = diazepam, EA = ellagic acid, Veh = vehicle. Control (Veh), received a single dose of EA (3, 10, 30 and 100 mg/kg, ip), Diazepam (1, 3 and 5 mg/kg, i.p.). Data reported as mean ± SEM and analyzed using one-way ANOVA test followed by post Hoc Tukey’s test.
Diazepam at dose of 1 mg/kg significantly decreased grooming compared with the control group, but had no significant effect on other open field parameters. Diazepam at doses of 3 and 5 mg/kg significantly decreased ambulation, rearing and grooming compared with the control group, but sniffing had no significant difference with the control group (Figure 3 A-D).
4.2.2. Effect of Chronic Administration of Ellagic Acid (30 mg/kg/day, 10 Days, orally by Gavage) on Motor Activity in Open Field Test
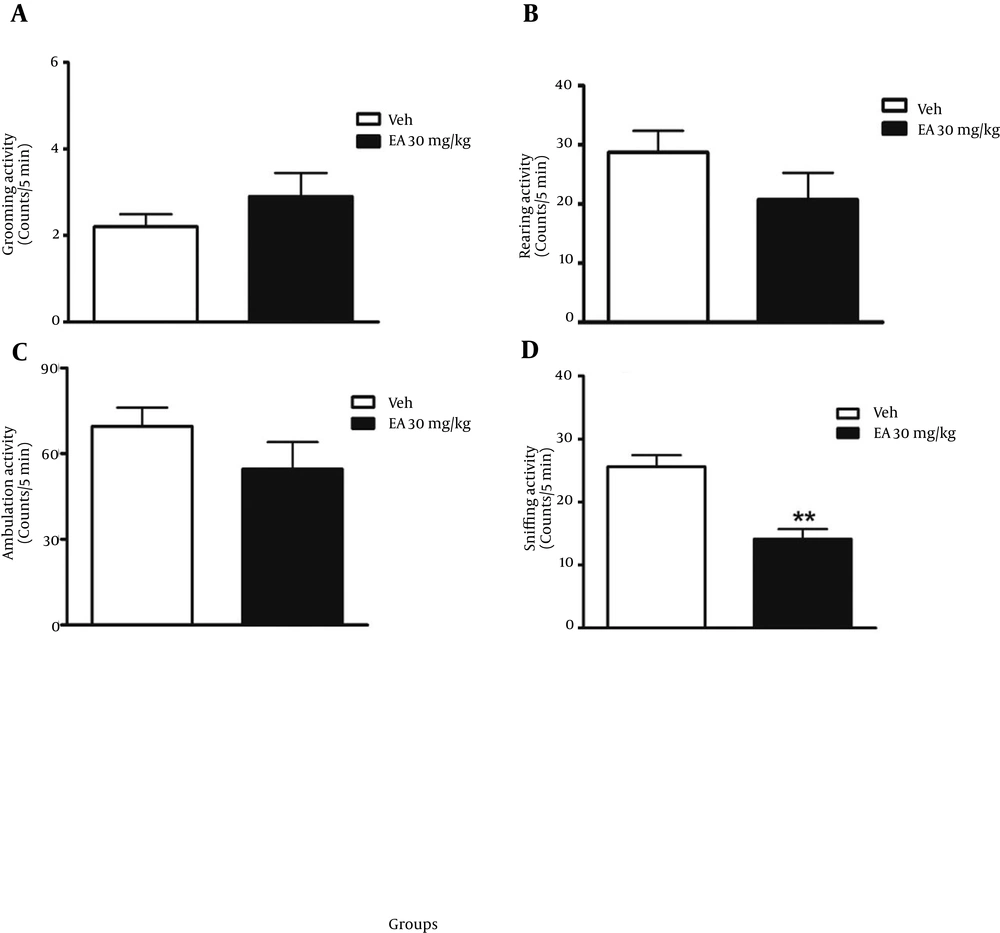
EA was administered orally by gavages for 10 days and 30 minutes after the last dose the animals were placed in open field and ambulation, rearing, sniffing and grooming activities were recorded. Results showed that ambulation, rearing and grooming had no significant differences compared with the control group, but sniffing decreased significantly compared with the control group (P < 0.01) (Figure 4 A-D).
A) Effect of 10 days of ellagic acid (30 mg/kg) administration on grooming activity in open field test (n = 8). B) Effect of 10 days ellagic acid (30 mg/kg) administration on rearing activity in open field test (n = 8). C) Effect of 10 days ellagic acid (30 mg/kg) administration on ambulation activity in open field test (n = 8). D) Effect of 10 days ellagic acid (30 mg/kg) administration on sniffing activity in open field test (n = 8). **P < 0.01. Abbreviations: EA = ellagic acid, Veh = vehicle. Data in all sections, A - D, reported as mean ± SEM and analyzed using one-way ANOVA test followed by post Hoc Tukey’s test.
5. Discussion
The aim of the present study was to evaluate the effect of different doses of EA (3, 10, 30 and 100 mg/kg) and chronic administration of EA (30 mg/kg) on anxiety and motor activity. The results demonstrated that EA at dose of 30 mg/kg had significant anxiolytic effect and at dose of 100 mg/kg had significant effect on motor activity in open field test. Co-administration of non-effective dose of EA (10 mg/kg) with non-effective dose of Diazepam (1 mg/kg) significantly decreased anxiety behavior in animals. This effect showed that EA and diazepam may exert their effects by a similar rout, as seen each of them alone had no effect on anxiety behavior of animals in EPM test. Co-administration of EA and Flumazenil significantly increased the number of entries in closed arm compared with the control group, which implies that EA exerts its anxiolytic effect via GABA A receptors, as Flumazenil is a GABA A receptor antagonist. Chronic administration of EA had no significant effect on anxiety and motor activity.
Flavonoids chemically are phenylbenzopyrones, generally conjugated with sugars and there are in all vascular plants. Numerous natural properties have been attributed to flavonoids. Amongst them antioxidant, anti-inflammatory, antihepatotoxic and antiviral activities are recognized, accompanied by vasculoprotective and spasmolytic effects. Flavonoids effects on the central nervous system have been measured only in the recent 10 years. Especially studies performed by Medina confirmed the ability of a number of flavonoids to attach the central type benzodiazepine (BZD) receptors (5).
GABAA receptor belongs to the ligand-gated ion channel superfamily. GABA is the most important inhibitory transmitter in the CNS. GABA binding to the GABAA receptor activates a chloride ion flux through the channel and BDZ-S ligands adjust the inhibitory effects of GABAA (20).
Several natural flavonoid compounds are found to be ligands for the γ-aminobutyric acid type A (GABAA) receptors in the central nervous system (CNS), which result in the theory that they act as benzodiazepine-like molecules (21, 22). These compounds have been shown to adjust GABA-generated chloride currents, either positively or negatively (21, 23).
Ellagic acid is a flavonoid compound that arises mainly as ellagitannins from plants such as raspberries, the stem and bark of eucalyptus species and nuts. This bioflavonoid has antioxidant, antifibrotic, anti-inflammatory, cardio-protective and anti-cancer properties (24). Girish et al. reported anti-depressant-like effect of ellagic acid, which is associated to its interaction with serotonergic and adrenergic system (25). Girish et al. assessed anxiolytic effect of ellagic acid. They used different doses from this study. Their results confirmed the present study. Nevertheless, they administered ellagic acid orally, in the present study ellagic acid injected intraperitoneally (4).