1. Background
Human desire to select the child's gender has historical roots. This issue has preoccupied human thought for long centuries. On the twentieth century, several studies were conducted in various parts of the world such as Germany, France, the United States, and the United Kingdom to partly satisfy this human desire through academic and experiential methods (1). Several factors such as cultural, social, religious, and economic issues, as well as medical reasons such as the existence of some diseases related to chromosome X encourage couples to choose traditional or new treatment methods for selecting their child’s gender (2, 3).
The establishment of family balance is another reason for selecting the gender of the child. In the past, when population control was not running in the country, most families were large and had children of both sexes. While with applying the population control policies, the number of children decreased and the ratio of boys to girls is relatively equal and balanced in the society; this ratio has not been established in the families. In this situation, most families have one male or female child and this issue may cause soreness. Therefore, some effective methods in this situation can promote the mental health of the community. Although in the normal condition, sperm determines the baby’s sex, factors affecting the choice of the sperm with the chromosome X or Y for oocytes fertility are not clear yet. Ericsson et al. believed that sperms containing chromosome Y due to less weight are more active than the sperms containing X chromosome (4). However, Rose et al. believed that enriching the sperm with cells containing Y chromosome is not the answer and other mechanisms can be involved (5). Generally, in human societies, the male to female ratio is balanced; but due to genetic differences in societies with different races, they have different male to female ratios. For example, the black people have the girl child more than the white people (6). Now there are several laboratory methods for determining fetal sex (7):
1, MicroSort is a sperm separation method in which sperms stained with fluorescent material are separated using flow cytometry device, and then through intrauterine insemination (IUI), those with the desired chromosome are transferred into the uterus. This method is widely used in animals (8); 2, pre-implantation genetic diagnosis (PGD) method in which after ovulation induction by microinjection, sperm is injected into oocytes and fertilization takes place. Next, using biopsy, a blastomer is separated from a 6 - 8 cell embryo and then by fluorescence-in-situ-hybridization (FISH) or molecular methods, blastomer’s gender is determined and after cultivation, the embryo with the desired gender is transmitted into the uterus (8); 3, a simple method of modified swim-up that is based on the movement of sperm and done within a few steps (9). Although, the success rate of this procedure to achieve male sex and female sex have been reported to be 89% and 86%, respectively; other researchers did not report a specific comment about the efficiency of this technique; 4, the gradient albumin method, which includes passing sperm from human albumin layers to separate the sperms containing Y chromosome in two different layers. It should be noted that the performance of this method in determining the gender of the child has been repeatedly discussed by the researchers. Some studies have confirmed the above method (4, 10, 11) and others have reported its failure (7, 12, 13). With regard to the paradoxical reports on the performance of this method, we decided to investigate the efficiency of albumen column using real time PCR method which is a good way to detect genetically different sperms.
2. Objectives
The study aimed to assess the separation of X- and Y-chromosome bearing sperms of fertile men on albumin gradients using real time PCR.
3. Patients and Methods
In this study, the sample was human semen. A total of 30 semen samples of the fertile people, referred to the IVF ward of Imam Khomeini hospital affiliated to Ahvaz Jundishapur University of Medical Sciences, were collected and studied after obtaining consents from the owners.
3.1. Examination of the Semen Samples
Semen samples of individuals referred to the IVF ward of Ahvaz Imam Khomeini hospital were tested to be healthy according to the guidelines of the world health organization (WHO), such as checking the number and percentage of the forward movements, in place and move on. Collected samples contained at least 30 million sperms and less than 20% abnormality. A sample of semen inside the sterile disposable container was collected and then, kept at 37°C for 15 minutes (14).
3.2. Gradient Albumin Method
In a Falcon pipe, 2 - 3 mL of semen and equal amount of Ham's F10 medium (Already hot) without albumen, was added and then blended at 2000 rpm for 10 minutes. Then, the surface liquid was discarded and 2 mL Ham's F10 medium was added to it. In order to separate the sperms containing X and Y chromosomes, gradient albumin was applied by the following procedure:
An amount of 1 mL of human albumin 10% was added to a small Falcon tube; then 0.5 mL sperm solution prepared from the previous stage was added and it was incubated at 37°C for 30 minutes. After 30 minutes, animated sperms got deposited and about 0.5 mL of sediment was removed. In another Falcon tube, 0.5 mL albumin 20% was poured and then 1 mL albumin 12.5% was slowly (drop by drop) added. Finally, 0.5 mL of the sperm solution from previous stage was added to this 0.5 mL and was incubated in an incubator at 37°C for 45 minutes. The deposited sperms (containing Y chromosome) are removed in each pipe with a pipette and centrifuged with the Ham’s F10 medium at 2000 rpm (for 2 times and each time for 5 minutes). Now, the sperms in the solution above the sediment contain X chromosomes.
Afterwards, the surface liquid was removed and 0.5 mL of the Ham’s F10 medium was added and the solution was kept until to do PCR technique at -20°C (14). Using the above method, all sperms of 20 samples were separated. The only sperm preparation Ham's F10 medium was done on the control samples so that was said. DNA was extracted from this separated sperms. For extraction of DNA of human sperm cells, Bioneer kit (AccuPrep® genomic DNA extraction kit. Cat. No.: K-3032) was used. With the help of a primer designed for SRY genes (which only exist in the sperms having Y chromosome) and HPRT gene (which is in the sperms having X chromosome) and ALBUMIN gene as the control gene (which there is only one version of it in all sperms of normal people) and doing the real time PCR method, SYBR green method, and analyzing with ∆∆Ct method, the percentage of sperms containing Y chromosome was measured. All data were analyzed by t test (using SPSS) and P < 0.05 was considered as significant level.
4. Results
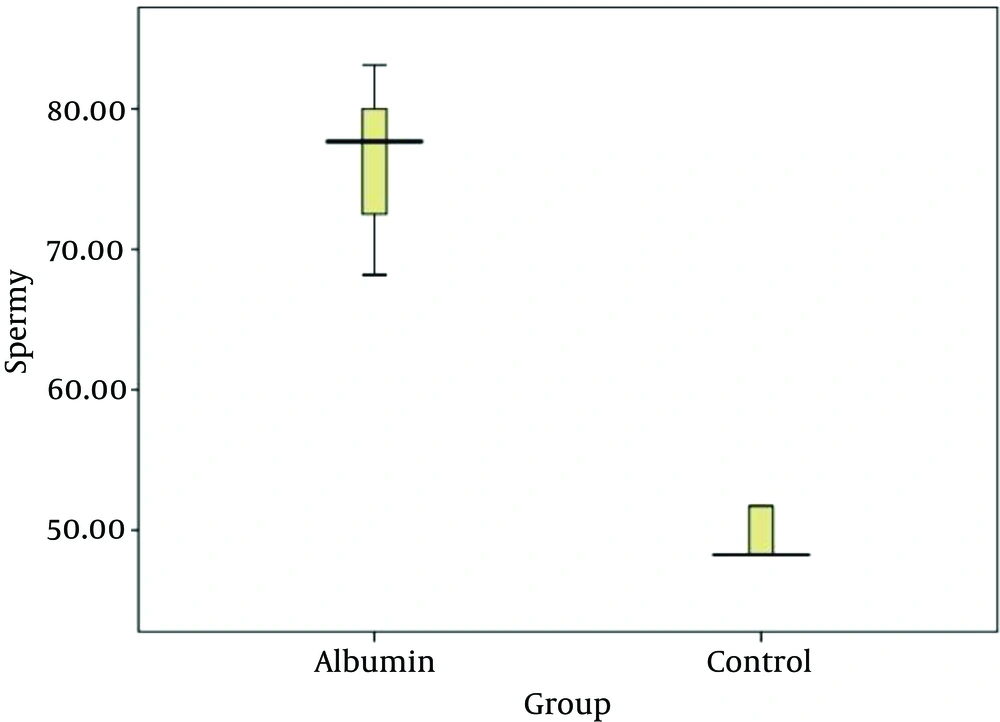
The average percentage of sperms carrying the Y chromosome in the albumin group (20 samples of human semen) was 75.80%, with the maximum and minimum values of 56.89% and 83.12%, respectively. The average percentage of sperms carrying the Y chromosome in the control group was 49.48% with the maximum and minimum values of 48.27% and 51.73%, respectively.
Using the t test, average amount of sperms having Y chromosome in the albumin group was compared with average amount of sperms having Y chromosome in the control group which was significantly different (P = 0.001) (Table 1). According to the mean values, the sperms carrying Y chromosome in the albumin group is more and indicates that albumen column caused passing and settling the sperms carrying Y chromosome. Therefore, the gradient albumin method has enriched the sperms containing Y chromosome.
Using the power analysis, the study power was obtained as 99% indicating an adequate sample size and with a higher power the achieved difference was more significant.
| Group | Number | Average Percentage of Sperms Carrying Y Chromosome | SD | T-Test | df | P-Value | Result of the Test |
|---|---|---|---|---|---|---|---|
| Albumen | 20 | 75.80 | 6.44 | 17.17 | 23.41 | 0.001 | Significant |
| Control | 10 | 49.48 | 1.64 | 17.17 | 23.41 | 0.001 | Significant |
5. Discussion
Prevention of sex-related diseases, selective abortion, physical lesions and psychological woes of mothers and families, overpopulation, as well as paying attention to the optimal social and psychological factors in balanced families, are the reasons for paying attention to the methods of gender selection before conception (15, 16). Today, there are several laboratory methods for determining fetal gender. One of these methods, which have been repeatedly investigated by researchers, is sperm separation method with the gradient albumin or Ericsson method which was published for the first time in the nature in 1973. In this method, the lighter Y sperms which move faster than the X sperms pass through the layers of human serum albumin and residue in the laboratory tube. This method is the first and most practical method to determine the gender of the fetus. Ericsson et al. reported that by doing this method, more than 85% of the Y-carrying spermatozoids were enriched (4). But in the studies conducted by, Ross et al. (5), Vidal et al. (7), to separate the sperms using gradient albumin and single lable FISH method, the above method was considered ineffective in the enrichment of the Y-carrying sperms (7, 17-21). Also in a study conducted by Wang et al. in 1994 using the double lable FISH method, it was reported that the gradient albumin did not enrich Y-chromosome carrying sperms (13). However, our research showed that by doing gradient albumin, the average percentage of Y-chromosome carrying sperms in the albumin group (20 samples of human semen) was 75.80% and the minimum and maximum values were 56.89% and 83.12%, respectively. The average percentage of Y-chromosome carrying sperms in the control group was 49.48% and the minimum and maximum values were 48.27% and 51.73%, respectively. About 75.80% of Y-chromosome carrying spermatozoids got enriched by this method, and this method is effective in determining the child's male gender and can be used for persons applying for fertility.
The study conducted by Rose et al. in 1998 about the efficiency of Ericsson method showed that out of 18 couples, who wanted the male child, 14 cases were successful and 4 cases had a miscarriage. Finally, the success rate for pregnancy with baby boy was over 80% (5). Pyrzak also believed that the above technique is effective to achieve the desired sex (especially male) (10, 11). In a study to achieve male child using Ericsson method, Esfahani et al. treated 45 couples. The results indicated that the achievement of the desired sex in the fertile couples was 80% (17). In a study conducted in 1997 using two methods of protocol 3 and modified protocol 3 albumin gradient and double lable FISH method, Flaherty et al. showed that no enrichment created in the Y-chromosome carrying sperms and the average ratio of X to Y sperm was 1.01 (14). In Jaffe et al. study, the success rate in achieving male gender and female gender was reported to be over 55% and 78%, respectively (18). In another study conducted by Silverman et al., clomiphene citrate was used during ovulation induction; the results showed that the baby's birth rate with girl gender was 74% (19). Likewise, in a study conducted by Khalili et al. to separate the sperms by albumin gradient method, it was shown that the rate of success in achieving the desired gender (male) was 71.4% (20). In addition, Corson et al. by using the technique for albumin isolation showed that 28 out of 35 cases of the pregnancy were male, and the success rate of this technique in achieving gender male was 80% (21). Check et al. reported similar results. They showed that with the use of the swim-up technique for the isolation of sperms, 81% of births were boys (22).
Nowadays other sensitive methods can be alternatives to Erickson method. However, the MicroSort technique, for example, by using flow cytometry device is not risk-free, let alone the cost of the relevant instruments and the imposition of heavy medical expenses to the couple (5). In MicroSort method, the sperms are stained with toxic fluorescent materials, under the x-ray machines that these factors are worrying. The application of this method had been evaluated more in animal reproduction as well as conditions such as genetic diseases dependent on X chromosome (23).
The PGD/FISH method is also very professional and expensive and due to the limitation of service centers, many of the applicants do not have access to it. However, this method has very high sensitivity for determining the gender (24, 25). Therefore, Ericsson method for sperm separation can be a successful method of determining the sex of the baby.
In conclusion, in this study, the average amount of sperms with the Y chromosome in the albumin group was more than that in the control group. This difference was statistically significant and indicates that the albumen column causes a lot of passing and settling the sperms carrying the Y chromosome and by using this method the sperms carrying the Y chromosome have been enriched up to 75.80%.
The results of this study showed that Ericsson method, by separating the sperms using an albumin gradient method, is a good and noninvasive method to determine gender in fertile couples. By employing the above method, the number of unwanted pregnancies or abortions decreases and diseases related to sex can be prevented.