1. Background
Exposure to stress has complex effects on learning and memory. According to the literature, stress can improve, destroy or have no effect on learning and memory (1, 2). These contradictory results can be explained by different variables, including the nature, duration and harrowing extent of the stressor, the extent and nature of the task and subject characteristics such as age and gender (3). Studies have shown that long-term stress can lead to cell death. It is believed that many of these structural changes following stress are involved in hippocampus function destruction, concerning spatial learning and memory, for example (4).
Studies have also shown that exercise can prevent the harmful effects of stress on the nervous system (5). There is evidence that physical activity can be effective in maintaining brain health. Short-term exercise increases memory and learning in young rats through improving the plasticity and neurothropic factors in the brain. The hippocampus, the most important center of learning and spatial memory, is also altered after exercise (6). The range of the effects of physical activity on cognitive function depends on the nature of the applied task and the type of exercise (7).
Exercise may be voluntary or forced. However, in most research, voluntary exercise was used (1, 8). It should be noted that the effects differ (9). For example, placing the mice in an enriched environment (in terms of activity), allowing them to work voluntarily, increases the production of neurons and enhances the performance of learning tasks related to the hippocampus (8). Exercise positively affects cognitive abilities. Treadmill exercise is an important factor for improving cognitive functioning (10).
It has been shown that subjects with moderate physical activity are less exposed to cognitive degeneration than inactive subjects (11, 12), but some studies have not shown this effect. For example, Mello et al. (13) studied the effect of vigorous physical activity for two weeks and long-term treadmill exercise combined with daily stress (due to a little stress associated with forced physical activity) for eight weeks on the different aspects of memory in rats. The results showed that stress and physical activity (severe or prolonged) do not affect the acquisition and retention of animals. Moreover, in both humans and animals, there were paradoxical results, possibly because of differences in exercise intensity and the duration of exercise protocols (14). Nakajima et al. (15) showed that voluntary exercise following eight weeks of physical restraint stress (two hours per day) increased IGF-1 and cell proliferation in the dentate gyros of the hippocampus and reduced the impairment of cognitive function. Zhang et al. (16) also stated that cardiovascular fitness resulting from physical activity increases anzyogenes, avoids excessive glutamate activity, protects the blood-brain barrier and prevents cell death that may be related to neuronal loss and stroke and improves the tolerance capacity of anemia.
However, according to some reports, exercise can be effective in older animals and has no effect in young animals (17). Research has also found that exercise has little effect in improving memory and does not affect object recognition tasks (13). While the majority of studies used voluntary exercise, in the current investigation, low-intensity forced exercise was used, which is similar to exercise for human studies where not well known (18). With forced exercise, time control and duration are easier than with voluntary exercise and volume control is possible (19). These fundamental differences between the two types of exercise may lead to different effects on the brain and behavior (20).
To cause stress responses, physical or psychological animal models of stress have been developed, from among which sleep deprivation stress has been accepted for experimental models in rodents (21). Studies in the case explicitly confirm the proposition that stress induced by sleep deprivation impairs cognitive factors such as memory and learning (21, 22).
In this case, as already mentioned, the effect of stress on learning and impact depends on duration, severity and age. In the present study, we tested learning and memory changes affected by stress and then modified by exercise. In other words, it is assumed that stress and its removal can lead to some changes in learning and memory. The stress of forced exercise after stress implication can also modify the negative effects. Therefore, the present study aimed to investigate the effect of exercise on learning and memory after REM in animal models. Thus, the survey results can clarify the role of sport as a factor in cognitive injuries.
2. Objectives
The purpose of this study was to investigate the moderating role of exercise in the negative effects of stress on learning and memory.
3. Materials and Methods
In this study, 16 one-year-old male Wistar rats weighing between 200 and 250 g were selected as the study sample. The rats were housed in collective cages (22 ± 2oC; 12 hours light-dark cycles) and fed on commercial laboratory chow (Labina, Ralston-Purina do Brasil, Ltd., Campinas, Brazil) ad libitum for one week and then progressed to the next processes. All stages of the study were approved by the Ethical Committee (approval number 93-1-16) for laboratory animals of the University of Shahid Chamran, Ahvaz, Iran.
3.1. Sleep Deprivation Protocol
For implication sleep deprivation, a modified multiple platform was used. The apparatus was comprised of 20 platforms with a height of 20 cm and a diameter of 5 cm. The distance between platforms was 7 cm, as in an aquarium. Water (24 ± 2°C) surrounded the platforms, and the water level was 1 cm below the edge of each platform. Each animal was placed on a platform with free access to food and water for 24 hours. It was reported that this method allowed animals to have general sleep but interfered with REM sleep. When muscle tone was lost during REM sleep, the rats fell into the water and the water caused the animals awaken (21-23). After implementing the sleep deprivation protocol, the rats were randomly divided into an exercise and control group (n = 8 rats for each group).
3.2. Exercise Protocol
The exercise group ran for seven days, with 30 minutes of treadmill exercise a day. In this protocol, the slope was zero and the training load was increased by elevating the speed in three phases: In the first 10 minutes, the speed was set to four meters per minute, in the second 10 minutes, the speed was increased to seven meters per minute and, in the third 10 minutes, the speed reached 10 meters per minute.
This type of low-intensity exercise was used to avoid any other stressful conditions that may lead to physical and physiological changes in animals (24). Controls were also placed on the off treadmill and there was no movement for this time. 24 hours after the end of the activity, the Morris water maze (MWM) test was used to measure the cognitive function in the rats.
3.3. Behavioral Tests
The MWM task was performed in a circular swimming pool similar to that described by Morris et al. (1982). The pool was made of black painted fiberglass, with a 1.7 m inside diameter and a height of 0.8 m, and was filled to a depth of 0.6 m with water maintained at 25 8 C. The target platform (10 cm - 10 cm) was made of transparent Plexiglas and was submerged 1 - 1.5 cm beneath the water surface. The starting points for the animals were marked on the outside of the pool as North (N), South (S), East (E) and West (W). Four distant visual cues (55 cm - 55 cm) were placed on the walls of the water maze room. They were all positioned with the lower edge 30 cm above the upper edge of the water tank, and the position of each symbol marked the midpoint of the perimeter of a quadrant (circle = NE quadrant, square = SE quadrant, cross = SW quadrant and diamond = NW quadrant). The apparatus was located in a room with indirect incandescent illumination. A monitor and a video-recording system were installed in an adjacent room. The animals were submitted to a spatial reference memory version of the water maze using a protocol that was adapted from one described previously by Ang et al. (2006). The training session consisted of seven consecutive trials, during which the animals were left in the tank facing the wall and then allowed to swim freely to the submerged platform. The platform was located in a constant position (in the middle of the southwest quadrant), equidistant from the center and the wall of the pool. If the animal did not find the platform during a period of 60 seconds, it was gently guided to it. The animal was allowed to remain on the platform for 10 seconds after escaping to it and was then removed from the tank for 20 seconds before being placed at the next starting point. This procedure was repeated seven times, with the starting points (the axis of one imaginary quadrant) varying in a pseudo-randomized manner. Latencies to find the platform and swimming speed were measured in each training session. The test session was carried out 24 hours later and consisted of a single probe trial in which the platform was removed from the pool and each rat was allowed to swim for 60 seconds in the water maze. The time spent in the correct quadrant (i.e. where the platform was located in the training session) and the swimming speed were also recorded (25).
3.4. Statistical Analyses
Data are presented as mean ± Standard deviation (mean ± SD). To determine the normal distribution of data, the Shapiro-Wilk test was used (because of the low size of the sample), and the test results showed the normality of the data (P > 0.05). A dependent t-test was used to compare each variable between the pre-test and post-test of each group. An independent t-test was applied to compare the MWM variables of the two groups one by one at the pre-test. It was also used to compare these variables between the two groups one by one at the post-test. The value of α = 0.05 was considered to be a significance level in all tests. Statistical analyses were performed by SPSS software version 16 for Windows.
4. Results
After subjecting the animals to sleep deprivation intervention for 24 hours, all animals (exercise and control groups) were induced to perform the MWM test to gain pre-test data. In this stage, to determine the normal distribution of data, the Shapiro-Wilk test was used (because of the low size of sample), and the test results showed the normality of the data (P > 0.05). The exercise group then proceeded to perform treadmill training.
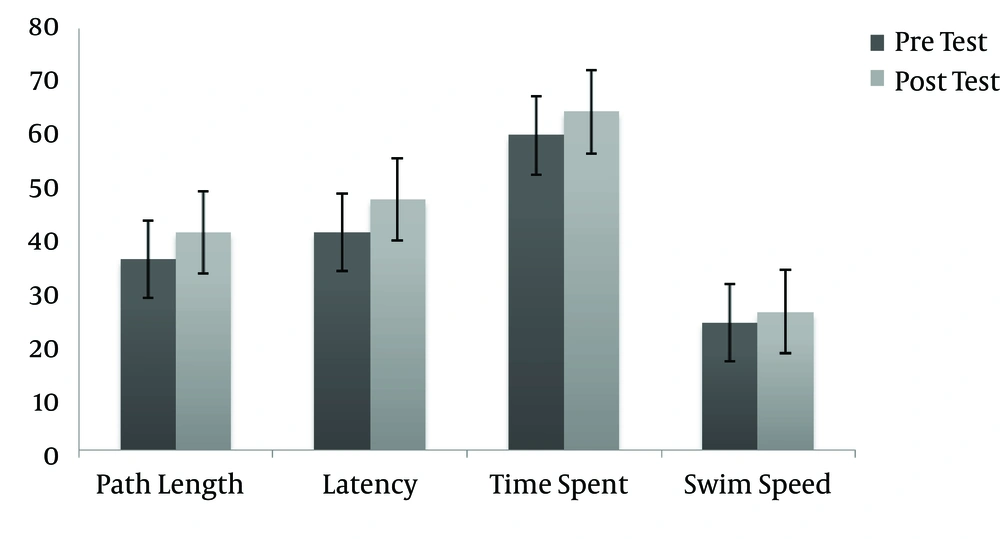
After one week of aerobic exercise by the exercise group, we tested learning and memory in the MWM again (acquisition) and compared them with the pre-training data. A dependent t-test was conducted on the data to compare the pre-test and post-test of each variable in each group separately. As shown in Table 1, The results of the dependent t-test for the exercise group showed significant differences in path length to probe (t = 2.283, P = 0.000), escape latency (t = 2.463, P = 0.00) and time spent in the platform area (t = 2.046, P = 0.03) and showed no significant difference in swim speed (t = 0.887, P = 0.058) after seven days of aerobic exercise on a treadmill (see Table 1 and Figure 1).
| Variable | Mean ± SD | Mean Diff | P | T (2, 17) |
|---|---|---|---|---|
| Path length to probe | 5.03 ± 1.00 | 0.000 | 2.283 | |
| Pre-test | 36.20 ± 4.12 | |||
| Post-test | 41.23 ± 3.12 | |||
| Escape latency | 6.12 ± 1.12 | 0.00 | 2.463 | |
| Pre-test | 41.33 ± 3. 33 | |||
| Post-test | 47.45 ± 4.45 | |||
| Time spent in target quadrant | 6.45 ± 6.64 | 0.03 | 2.046 | |
| Pre-test | 59.67 ± 7.23 | |||
| Post-test | 64.12 ± 6.59 | |||
| Swim speed | 2.12 ± 2.23 | 0.058 | 2.010 | |
| Pre-test | 24.12 ± 3.33 | |||
| Post-test | 26.24 ± 1.10 |
These tests were also performed on the control group. The results showed no significant differences between the pre- and post-test values of path length to probe (t = 1.155, P = 0.000), time spent in the platform area (t = 0.246, P = 0.9), escape latency (t = 0.463, P = 0.17) and swim speed (t = 0.887, P = 0.21) in the control group (P > 0.05) (see Table 2).
| Variable | Mean ± SD | Mean Diff | P | T (2, 17) |
|---|---|---|---|---|
| Path length to probe | 0.10 ± 1.03 | 0.42 | 0.981 | |
| Pre-test | 36.10 ± 3.9 | |||
| Post-test | 36.20 ± 4.12 | |||
| Escape latency | 0.29 ± 1.04 | 0.17 | 0.463 | |
| Pre-test | 41.62 ± 4.29 | |||
| Post-test | 41.33 ± 3.33 | |||
| Time spent in target quadrant | 1.10 ± 1.12 | 0.09 | 0.246 | |
| Pre-test | 58.87 ± 8.11 | |||
| Post-test | 59.67 ± 7.23 | |||
| Swim speed | 0.43 ± 1.25 | 0.21 | 0.887 | |
| Pre-test | 24.65 ± 4.58 | |||
| Post-test | 24.12 ± 3.33 |
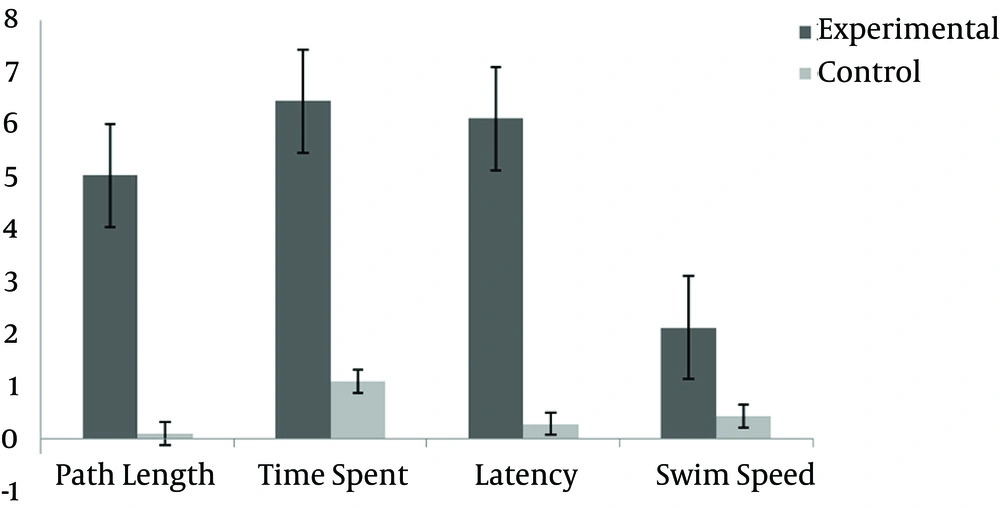
Furthermore, when comparing the post-test results of the two groups, because of the high correlations between variables, we first calculated the mean differences of four variables in each group and then applied an independent t-test to compare each post-test variable of the two groups. The results showed significant differences between the two groups in three variables of the MWM: path length (t = 2.283, P = 0.001), time spent in the platform area (t = 0.463, P = 0.00), escape latency (t = 2.046, P = 0.03) and swim speed (t = 2.887, P = 0.02) (Table 3 and Figure 2).
| Variable | Mean ± SD | Mean Diff | P | T (2, 17) |
|---|---|---|---|---|
| Path length to probe | 0.10 ± 1.03 | 0.001 | 1.798 | |
| Experimental | 5.03 ± 1.00 | |||
| Control | 0.10 ± 1.03 | |||
| Escape latency | 0.29 ± 1.04 | 0.000 | 1.781 | |
| Experimental | 6.45 ± 6.64 | |||
| Control | 1.10 ± 1.12 | |||
| Time spent in target quadrant | 1.10 ± 1.12 | 0.03 | 2.046 | |
| Experimental | 6.12 ± 1.12 | |||
| Control | 0.29 ± 1.04 | |||
| Swim speed | 0.43 ± 1.25 | 0.02 | 2.281 | |
| Experimental | 2.12 ± 2.23 | |||
| Control | 0.43 ± 1.25 |
These findings indicate that, 24 hours after training (the acquisition phase), seven days of aerobic exercise on a treadmill leads to better performance in cognition and learning in sleep deprivation stress-induced animals.
5. Discussion
A large and growing body of literature confirms that sleep deprivation causes stress in humans and animals. Therefore, the results of induced stress are not shown in this paper, and we strongly assume that 24 hours of sleep deprivation significantly increased stress. The pre-test results showed that there were no significant differences between memory and learning in the MWM test.
The results showed that 24 hours of sleep deprivation stress impaired learning. This result was confirmed by increasing the time and distance to reach the platform in the acquisition phase. In this regard, there is evidence that stress and exposure to glucocorticoids induces dendritic atrophy in the hippocampus by nerve damage associated with reduced neurogenesis (26) and is involved in neuronal plasticity changes. Such changes in the hippocampus are known as a cognitive mechanism underlying stress exposure and are generally attributed to changes in corticosterone.
However, in the retention phase, the destructive effect of stress was not observed. To explain the different effects of stress in the acquisition and retention phases, the causes may be related to the fact that, in the present study, the acquisition test was conducted one day and the retention test was performed nine days after the end of stress implication (27-29). It is assumed that the animals returned to a normal condition during this period. To support this hypothesis, studies have shown that, while eliminating stress, stress-induced changes in the hippocampus gradually return to the initial state (30).
Researchers have shown that regular exercise through various physiological adaptations, such as metabolic rate, mitochondrial biogenesis and adaptation of the HPA axis, to respond to environmental change provides for the organism (31). In contrast to our findings, a study by Kim et al did not show such effects (32), but their exercise protocol was done for eight for a duration of eight weeks spent running on a treadmill with a higher intensity than the present study.
Evidence is available that has shown that, by increasing the training period (from nine days to 24 days), HPA axis activity in rats is gradually increased (33). Thus, it can be concluded that the normal function of impaired spatial learning and memory caused by stress may be related to physical activity. Exercise protects against the negative effects of stress on learning and memory. These findings are in agreement with the results of Kim et al and Reisi et al. (32, 34).
The reports found that running reduced impaired cognitive function through neurogenesis in the dentate gyrus of the hippocampus (35) and can cope with the effects of stress (36). It seems that the results of initial training may have nervous protection features against stress-induced sleep deprivation. The absence of a significant difference in the speed at which the animals reach the target indicates that stress or exercise does not affect the transition behavior of the subjects.
In this study, a one-week training program on a treadmill had no effect on spatial learning in the acquisition phase. These findings are in line with those of O’Callaghan et al. (33) and Reisi et al. (34), which showed that spatial learning is not influenced by exercise.
This inconsistency may be due to differences in the length and intensity of the exercise protocol. The results of Babri et al. (37) showed that cell proliferation in the dentate gyros of rats is regulated by the intensity and duration of exercise, which shows that the exercise protocol may have significant effects on neural function. However, due to the inherent stress of forced exercise, it is assumed that this type of exercise may not induce beneficial effects in acquisition, but this hypothesis is somewhat challenging. Some reports suggest that moderate treadmill training can improve spatial learning in aged rats (38).
In research by Raeisi and Akalaqan, the exploration retention test (probe) was used. This test is performed 24 hours after the acquisition phase. In a study by Saadati et al. (39), cognitive function was measured by avoidance learning, and it was found that the difference between test types may lead to different results in evaluation exercise effects on memory.
Various protocols of differing intensities of exercise can also lead to different effects on the neurological function (40). Furthermore, based on learning definition, relatively stable are as the learning aspects. Thus, with regard to the length of the delay between acquisition and retention (four days in this study), which was longer than other studies (24 hours), this is closer to the learning definition.
It is clear that the ability to remember past experiences in the long term is consistent with learning, and remembering experiences at long intervals indicates better memory and efficiency of cognitive function than in shorter periods.
The retention test was performed 10 days after the end of the exercise protocol. The results are in agreement with those of the study by Berchtold et al. (40). They showed that the effects of exercise on cognition persist even after the cessation of exercise. The researchers found that it took those animals that had between one and two weeks of rest between the end of exercise and cognitive tests less time to reach the podium (41).
Similar improvements in both acquisition and retention performance were not seen in all studies. The effects of exercise on cognitive processes may be dependent on factors such as duration, type of exercise (forced or voluntary), task complexity and other factors that are not well known. In addition, neural adaptation is triggered despite the activation of the HPA. Accordingly, exercise can be used as a tool in the prevention of neural traumatic events and cause improved performance. Additionally, even with the elimination of exercise, the positive benefits on cognition remain for several weeks. The results are useful for illness or injury or for special conditions of people unable to participate in sports and can be a way to avoid performance decline.
Stress induced by sleep deprivation can cause negative physical, psychological and cognitive effects, and moderate and regular exercise can be applied to deal with stress. The present findings show that exercise, even in the short term, can modify cognitive impairments induced by sleep deprivation and stress and improve learning and memory in rats. These results can be cautiously generalized to human subjects.