1. Background
Metabolic syndrome (MS) or X syndrome is a cluster of cardiovascular risk factors including abdominal obesity, high blood pressure, abnormal glucose and lipid metabolism (high triglyceride, low high-density lipoprotein (HDL) cholesterol), and high blood glucose (1). The prevalence of MS in different areas of the world is 12.8% - 41.1% (2). According to different studies, a high percentage of the Iranian population is afflicted with MS in different provinces (21.9% - 31.1%) (1, 3-5).
Considering the fact that MS is highly correlated with diabetic and cardiovascular risk factors (1), and predisposes the afflicted person with a higher incidence of stroke, micro-albuminuria, neuropathy, retinopathy, and other co-morbidities (1), a treatment with appropriate remedies is a fundamental step in the management of MS, cardiovascular diseases, and diabetes (1). Dietary interventions are among the therapeutic approaches responsible for affecting prominent components of MS such as abdominal obesity, high blood pressure, impaired glucose tolerance, dyslipidemia, inflammation, and oxidative stress. Flavonoid-rich foods are among the therapeutic dietary interventions (6). Polyphenols are one of the dietary natural components responsible for health promotion in many chronic diseases, which can be an adjunctive therapy for MS as well. Cranberry is among the fruits with the highest phenolic content and has a high antioxidant activity (7). The most important polyphenols present in cranberry are flavonoids and ellagic acid (8, 9). Cranberry possesses antioxidative, anti-inflammatory, anti-hypertensive, anti-hyperlipidemic, and anti-diabetic properties (7, 10). In addition, low-density lipoprotein (LDL) oxidation and platelet aggregation can be inhibited through cranberry consumption due to its phytochemical content. It can also induce the LDL receptor and reduce blood pressure (11-14) and can have an independent effect on preventing HDL reduction, and therefore might impose a cardio-protective effect (15). Therefore, consuming cranberry might favorably affect the components of MS (10).
2. Objectives
Convincing evidence on beneficial effects of cranberry on glucose and lipid metabolism is not available and this entails more studies about this fruit and its products (16). Hence, to delineate the possible effects of cranberry on MS components, we assessed the effects of cranberry supplementation on metabolic risk factors in obese and overweight females.
3. Patients and Methods
This was a randomized, placebo-controlled clinical trial conducted in accordance with the declaration of Helsinki and good clinical practice guidelines. Informed consents were obtained from all the participants. The study protocol was reviewed and approved by the ethics committee of Shiraz University of Medical Sciences.
Forty eight obese and overweight females (aged 18 to 60 years) were recruited at the health center at Shiraz University of Medical Sciences. Eligible participants had waist circumference of 88 cm or above and a body mass index over 25 kg/m2. The exclusion criteria included lactation, pregnancy, chronic diseases such as coronary heart disease, diabetes and etc., renal, hepatic or endocrine abnormalities, tobacco use, taking antioxidant, flavonoid, or fish oil supplements, and medications affecting lipid or insulin metabolism or possessing anti-inflammatory effects.
The sample size was determined using the result of a previous study (16). A sample size of 21 participants in each group was determined according to the total cholesterol difference and with SD of 1, α = 0.05 and power of 80% of the total number of participants before the dropouts in each group. Therefore, the final sample size was 24 participants in each group.
After screening 150 patients with MS at the health centers of Shiraz University of Medical Sciences, 42 people were found to be eligible to participate in the trial. Blocked randomization with a fixed block size of four was performed using Random Allocation software to assign the participants into two groups. The participants in the first group took two capsules of cranberry extracts daily for eight weeks (one capsule after lunch and another one after dinner). The dose of cranberry supplementation was 400 mg/day. We selected the cranberry dose based on a previous berry intervention that showed cardiovascular health benefits (16). Nutrient analysis of each of the two capsules was as follows, energy 9 kJ, protein 0.15 mg, sugar 0.34 mg, lipid 0.01 mg and proanthocyanidins 12 mg. The cranberry supplements were supplied by Vitarmonyl Laboratories.
Those in the second group took one capsule of placebo daily for eight weeks. The placebo capsules were provided by the School of Pharmacy at Shiraz University of Medical Sciences and were made of cellulose in pre-packed bottles. The placebo and cranberry capsules were similar in size, color, weight, and taste. All the investigators and health care staff were blinded to the treatment assignment.
All the subjects were asked to maintain their usual dietary habits, physical activity, and lifestyle during the eight-week period of the study. We recorded the consumption of flavonoid-rich foods using a food frequency questionnaire (FFQ) specific for flavonoids, and measured body weight, height and waist circumference by standardized methods (17). Blood pressure was measured by a manual sphygmomanometer after a five-minute rest between two blood pressure measurements.
After 12 hours of fasting, blood samples (5 mL) were collected at the beginning and end of the treatment phase (eight weeks). Serum samples were separated by centrifugation at 3000 rpm for 10 minutes and stored at -70ºC. Upon collection, serum glucose and lipoproteins were determined by enzymatic methods (calorimeter) using a Biosystem A25 analyzer. We measured serum malondialdehyde (MDA) by the modified thiobarbituric acid method (spectrophotometric method) (18) and used commercially available ELISA kits to determine serum concentration of high-sensitivity C-reactive protein (hs-CRP) (IBL international GmbH, Flughafenstrasse 52a, D-22335 Germany), adiponectin (Boster Biological Technology Ltd), IL-6 (AviBion, Orgenium Laboratories), and insulin (Monobind Inc., Lake Forest, CA 92630, USA). We evaluated the insulin resistance by homeostasis model assessment calculated as:

Statistical analysis for social sciences (SPSS) version 16 was used for statistical analyses (SPSS Inc., Chicago, IL). We analyzed normal distribution of continuous variables by Kolmogorov-Smirnov Test. Paired student t-test was used to determine the significance of metabolic changes in each group. The difference between the control and cranberry groups at week eight was assessed using independent t-test for normally distributed data. Skewed data were compared using Mann-Whitney U-test and Wilcoxon ranked test. P values less than 0.05 were considered statistically significant. The data were presented as means ± standard deviations.
The study was conducted with the IRCT registration number of IRCT201110192480N2.
4. Results
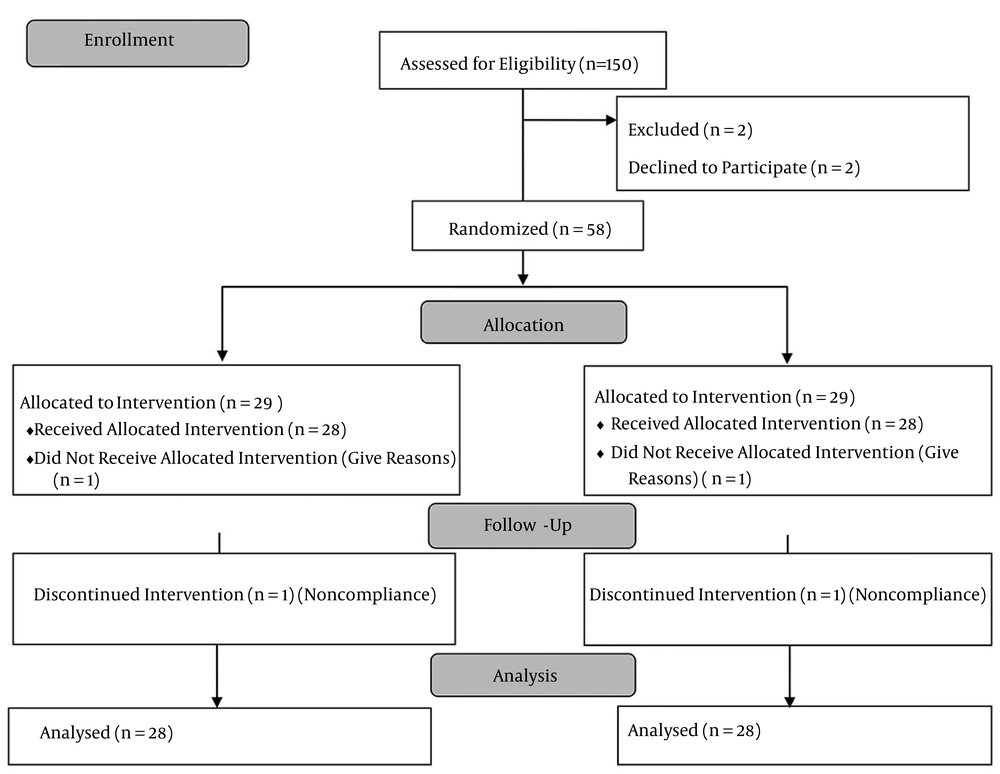
Eligible participants were recruited from January 2012 to April 2012. Figure 1 shows the flow diagram of the study, and accordingly, one participant was excluded in the cranberry group and one in the placebo group. Table 1 shows the baseline demographic and laboratory characteristics. No statistically significant differences were seen in the baseline characteristics. Table 2 shows the characteristics of the two groups at the baseline and week eight of the study. HDL cholesterol increased significantly in the cranberry group (P = 0.049). The changes in HDL cholesterol were significantly different between the groups (P < 0.013). In contrast, serum total and LDL cholesterol levels did not change significantly between the two groups. Triglyceride levels statistically decreased in the cranberry group, but the change was not significant between the two groups (Table 2). A decreasing trend in waist circumference (P < 0.001) and systolic blood pressure (P = 0.015) was observed in the cranberry group. Changes in other measurements were not statistically different between the two groups (Table 1).
| Demographic and Laboratory Characteristics | Cranberry Supplement | Placebo | P Value |
|---|---|---|---|
| Age, y | 42.4 ± 5.3 | 41.7 ± 6.5 | 0.702 |
| BMI | 29.2 ± 3.5 | 29.5 ± 3.3 | 0.77 |
| SBP, mmHg | 117.83 ± 0.81 | 118.12 ± 0.83 | 0.936 |
| DBP, mmHg | 79 ± 7.95 | 78.75 ± 10.85 | 0.928 |
| HDL, mg/dL | 48.37 ± 8.1 | 45.25 ± 9.94 | 0.24 |
| LDL, mg/dL | 110.67 ± 26.53 | 111.67 ± 34.07 | 0.91 |
| TG, mg/dL | 173.42 ± 80 | 196.25 ± 113 | 0.424 |
| Chol, mg/dL | 203.08 ± 38.15 | 219.46 ± 81.06 | 0.375 |
| WC, cm | 93.47 ± 7.23 | 91.93 ± 7.26 | 0.465 |
| FBS, mg/dL | 114.12 ± 33.99 | 111.33 ± 34.23 | 0.778 |
| Insulin, µIU/mL | 10.32 ± 8.18 | 9.54 ± 6.69 | 0.718 |
| IL-6, pg/mL | 10.29 ± 6.35 | 14.53 ± 8.44 | 0.056 |
| Adiponectin, ng/mL | 16.91 ± 3.26 | 13.11 ± 2.41 | 0.548 |
| hs-CRP, µg/mL | 5.34 ± 2.50 | 7.41 ± 3.53 | 0.61 |
| MDA, µM | 2.13 ± 0.81 | 2.96 ± 0.83 | 0.084 |
aThe data are expressed as mean ± SD, the categorical data are expressed as numbers.
| Variable | Cranberry (N = 24) | Placebo (N = 24) | ||
|---|---|---|---|---|
| Wk 0 | Wk 8 | Wk 0 | Wk 8 | |
| Systolic blood pressure, mmHg | 117.83 ± 11.19 | 113.75 ± 11.72 | 117.61 ± 11.76 | 116.52 ± 13.93 |
| Diastolic blood pressure, mmHg | 79.00 ± 7.95 | 77.91 ± 9.99 | 78.26 ± 10.83 | 79.13 ± 11.93 |
| Glucose, mg/dL | 114.12 ± 33.99 | 118.7 ± 40.06 | 111.5 ± 34.98 | 106.17 ± 21.37 |
| Triglycerides, mg/dL | 173.42 ± 80.03 | 144.29 ± 49.14 | 179.00 ± 76.78 | 179.39 ± 89.41 |
| Total cholesterol, mg/dL | 203.08 ± 38.15 | 206.92 ± 38.23 | 204.65 ± 37.00 | 198.78 ± 40.48 |
| LDL cholesterol, mg/dL | 110.67 ± 26.53 | 114.12 ± 28.58 | 108.22 ± 30.25 | 106.87 ± 23.30 |
| HDL cholesterol, mg/dL | 48.37 ± 8.13 | 51.00 ± 8.22b | 45.60 ± 10.01 | 45.00 ± 7.69 |
| Insulin, µIU/mL | 10.32 ± 8.18 | 8.40 ± 5.25 | 9.46 ± 6.84 | 11.40 ± 10.20 |
| HOMA-IR | 3.00 ± 2.77 | 2.55 ± 2.09 | 2.59 ± 2.00 | 3.22 ± 3.56 |
| MDA, µm | 2.13 ± 081 | 2.17 ± 0.72 | 3.02 ± 0.81 | 3.01 ± 0.74 |
| hs-CRP, µg/mL | 5.34 ± 2.50 | 5.27 ± 2.87 | 7.56 ± 3.54 | 5.08 ± 2.49 |
| IL-6, pg/mL | 10.29 ± 6.35 | 9.35 ± 6.57 | 15.10 ± 8.45 | 9.20 ± 5.62 |
| Adiponectin, ng/mL | 16.91 ± 3.36 | 16.93 ± 3.41 | 13.18 ± 2.44 | 14.30 ± 3.06 |
| Waist circumference, cm | 93.47 ± 7.23 | 91.47 ± 7.17 | 91.28 ± 6.66 | 90.21 ± 6.18 |
aValues are expressed as mean ± SD.
bIt was significantly different between cranberry and placebo group at week eight (P < 0.05).
5. Discussion
Our study demonstrated the positive effect of cranberry supplementation on HDL-c. Few interventional studies have shown the effect of cranberry on HDL-c. This effect might be due to an elevation in apo A-I and a decrease in HDL particles clearance that is mediated by cranberry supplements, or in other words, by polyphenolic compounds (7, 8, 14, 19-29). On the other hand, quercetin, one of the most prominent flavonols of cranberry, can increase HDL-associated enzymes such as paraoxanase-1 (PON-1) (30). This enzyme can increase the circulating HDL-c through stimulating macrophage cholesterol efflux (1). The potential effect of cranberry on serum HDL-c was not seen in another study due to some limitations (16) including study duration and sample size.
High oxidative stress (30) and elevated inflammatory markers such as IL-6 and tumor necrosis factor-α (TNF-α) (26, 31) are implicated in people with MS. On the other hand, anti-inflammatory markers including adiponectin reduce in the course of the abnormalities of MS (32). MDA is a stable biomarker of lipid peroxidation and is highly correlated with MS (33, 34). The effects of cranberry flavonols on elevating the paraoxonase 1 (PON-1) activity has been proposed to reduce inflammation and oxidative stress (15). In addition, PON-1 is responsible for reducing monocyte adhesion to endothelial cells and this can alleviate phospholipid oxidation through the management of macrophage chemotaxis (35-37). Moreover, berry extracts are effective in reducing inflammation in cellular models (38). Quercetin in cranberry is a potent down-regulator of nuclear factor kappa B (NFK-B) pathway, through which it can reduce inflammatory markers, especially IL-6 (39). Therefore, the effects of cranberry supplements on inhibiting the aggravation of oxidative stress and inflammation are obvious (15). In a short-term intervention by Basu and his colleagues, a decrease in MDA concentration following the consumption of cranberry juice was seen in people with MS after eight weeks (16). Additionally, in other studies in healthy volunteers, the beneficial effects of cranberry supplements on oxidative stress were also observed (30, 40). All the results of the mentioned studies are inconsistent with the result of the present study, as no significant effect of cranberry supplement was seen on serum MDA in the current study. Additionally, with regard to the effects of cranberry supplements on MDA, the same results as ours were observed in a study by Duthie (41). Perhaps, higher doses of cranberry supplements or other strategies such as life-style changes in combination with intervention are needed to further investigate the effects of cranberry supplements on oxidative stress in those with MS.
Regarding the effects of cranberry supplements on serum levels of IL-6, hs-CRP and adiponectin as inflammatory markers, no significant differences were found between the cranberry supplement and placebo-consuming groups in the current study. This is in accordance with the results of a study by Basu on people with MS (16). The differences regarding inflammatory markers may reach significant levels with larger sample sizes or longer durations.
Finally, about the features of MS, cranberry supplement showed no significant effect on waist circumference, blood pressure or insulin resistance after eight weeks, and that was in line with the results of a study by Basu (16); while, in some trials, improvements in blood pressure or insulin resistance were seen following cranberry extract consumption (42, 43).
The main limitations of our study were the short duration of the study and the low sample size. In addition, including only females in our study might have had an impact on the results.
To sum up, the findings of our study demonstrated the efficacy of cranberry supplement in improving HDL-c in obese and overweight females with MS in short term, but it could not affect other features of MS or inflammatory and oxidative markers. Therefore, further trials with larger sample sizes and longer durations are needed to investigate the efficacy of this supplement and to delineate its possible mechanisms of action.