1. Background
Uterine cancer remains the most common gynecologic malignancy in developed countries with an estimated 40100 new cases and 7470 deaths in the US in 2008 (1). Endometrial cancer comprises approximately 95% of the cancers of the uterine corpus. The majority of patients present the stage I disease, which has a cancer-specific survival rate approaching 90% (2). Recurrence of uterine cancer can occur regardless of the initial clinical staging and is associated with significantly poorer outcomes. The risk of recurrence depends on prognostic factors, such as stage at initial diagnosis, tumor differentiation, depth of myometrial invasion and lymph node involvement. Three-year survival rate following vaginal, pelvic and distant recurrence are thought to be 73%, 14% and 8%, respectively (3).
With increasing emphasis on individualized cancer care, predicting individualized postoperative outcomes based on readily available clinical and pathological information may add value to medical decision-making by providing an accurate prediction of survival, and thus helping guide follow-up strategies.
2. Objectives
The aim of this study was to evaluate the five-year survival rates, prognostic factors and recurrence patterns of patients with endometrioid-type endometrial cancer.
3. Patients and Methods
Patients with early stage endometrioid-type endometrial cancer (n = 102) who had been treated at the gynecology oncology ward of Vali-e-Asr Hospital, Tehran, Iran, between years 1999 and 2009, were evaluated. The diagnosis of early or low-risk disease was made on the basis of preoperative diagnostic pathologic evidence and clinical findings; preoperative imaging was done only for selected patients at the discretion of individual practitioners.
The primary surgery was performed by a gynecologic oncologist. All patients had a total abdominal hysterectomy, bilateral salpingo-oophorectomy, and lymph node sampling. Recommendations for adjuvant treatment (either radiation or adjuvant chemotherapy with or without radiation) were usually reserved for those with a moderate to high risk for recurrence. The chemotherapy regime used was paclitaxel, doxorubicin (adriamycin) and cisplatin.
The patients who had a history of concomitant or previous malignant disease, received treatment elsewhere, incomplete follow-up data and a histopathologic diagnosis other than endometrioid type endometrial carcinoma, as well as those who did not undergo initial surgical intervention including pelvic-paraaortic lymph node dissection were not included in this study. Data was obtained from special oncology files and the following features were recorded for each patient: age at diagnosis, parity, surgical procedure, sub-stage, histology, grade, depth of myometrial invasion, size of tumor, lymph vascular space invasion, lymph node status, adjuvant therapy, surgical morbidity, time to recurrence, date of death, or last follow-up. Patients were followed every three months for the first two years, and every six months thereafter. Median follow-up period was 30 months (range, 3 - 108).
Statistical analysis was performed using SPSS Base 18.0. The objectives for this analysis were to evaluate prognostic factors and to assess the outcomes that were associated with treatment. End points included recurrence and survival. Univariate analysis associated with survival was performed by the Kaplan–Meier method. disease-free survival (DFS) was calculated from date of diagnosis to date of recurrence, death, or last follow-up. Overall survival (OS) was calculated from date of diagnosis to date of death or last follow-up. The log-rank test was used to compare survival curves. Multivariate analysis was performed by Cox's regression analysis to assess the significance of prognostic factors. Probability values of < 0.05 were considered statistically significant.
4. Results
The demographic and histopathologic characteristics of the 208 patients who where included in the final analysis are presented in Table 1. The mean age at the time of diagnosis was 54 years (range, 28 - 74). Multivariate analysis revealed that age was a significant independent predictor of survival (P = 0.015). In the final pathologic report 22 (10.8%) patients had no myometrial invasion, 120 (58.8%) had invasion of less than one-half of the myometrial thickness, and 62 (30.4%) had greater than one-half of the myometrial invasion. Grade 2 was the most common histopathologic grade, seen in 100 patients (49%), while grade 3 was present in 30 patients (14.7%). Twenty-six patients (12.7%) had lympho-vascular space invasion (LVSI) and 178 (87.3%) of the tumors demonstrated no LVSI. Furthermore, 144 (70.6%) cases were in stage I at diagnosis and 20 (9.8%) cases were in stage II. Thirty patients had involvement of the serosa and adnexa and/or positive peritoneal cytologic findings (14.7%; stage ШA), two patients had involvement of the vagina (1%; stage ШB), and six patients had pathologically-confirmed positive pelvic and/or paraaorttic lymph nodes (2.8%; ШC). Pelvic node metastases were identified in 1% (2/208), aortic node metastases in 1% (2/208) and both pelvic and aortic node metastases were identified in 1% (2/208) of the patients.
| Characteristics | Values |
|---|---|
| Myometrial invasion | |
| ≤ 50% | 69.6 |
| > 50% | 30.4 |
| Architectural grade | |
| G1 | 36.3 |
| G2 | 49 |
| G3 | 14.7 |
| Lymph-vascular space involvement | |
| Nill/Minimal | 87.3 |
| Moderate/Prominent | 12.7 |
| Cervical invasion | |
| Negative/Cervical gland | 98 |
| Stromal invasion | 2 |
| Peritoneal cytology | |
| Negative | 85.3 |
| Positive | 14.7 |
| Ovarian metastasis | |
| Negative | 82.3 |
| Positive | 17.7 |
| Lymph node metastasis | |
| Negative | 97.2 |
| Positive | 2.8 |
aValues are presented as percent.
Seventy (34.7%) patients were treated with external beam radiotherapy (EXT) and intracavitary vaginal cuff brachytherapy (VB), 18 (8.9%) with VB alone and 32 (15.8%) with chemotherapy combined with radiotherapy. No patient received pre-operative EXT. Mean follow-up duration was 34 months (3 - 108) for all patients.
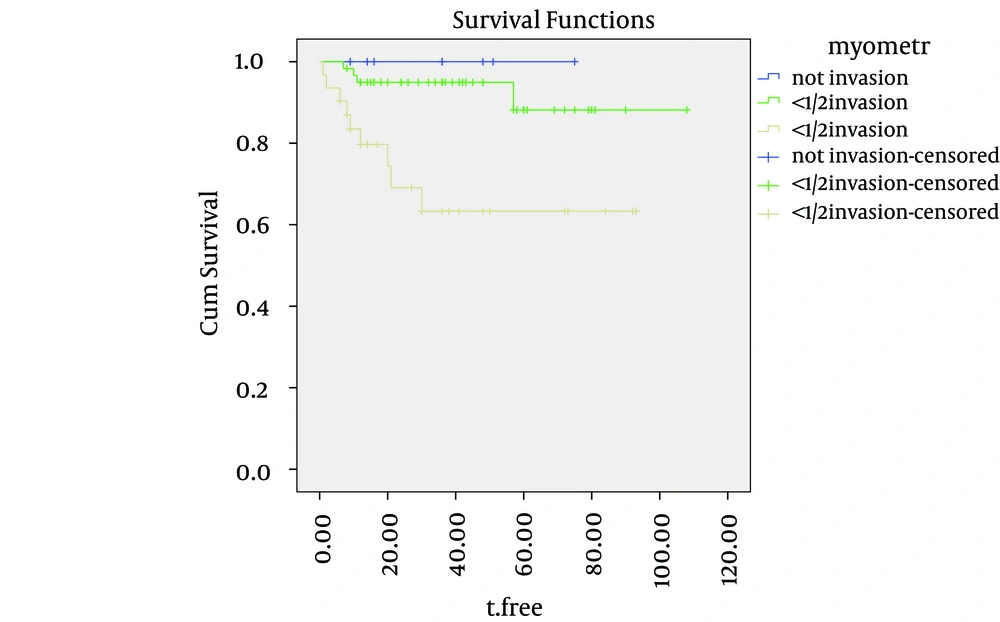
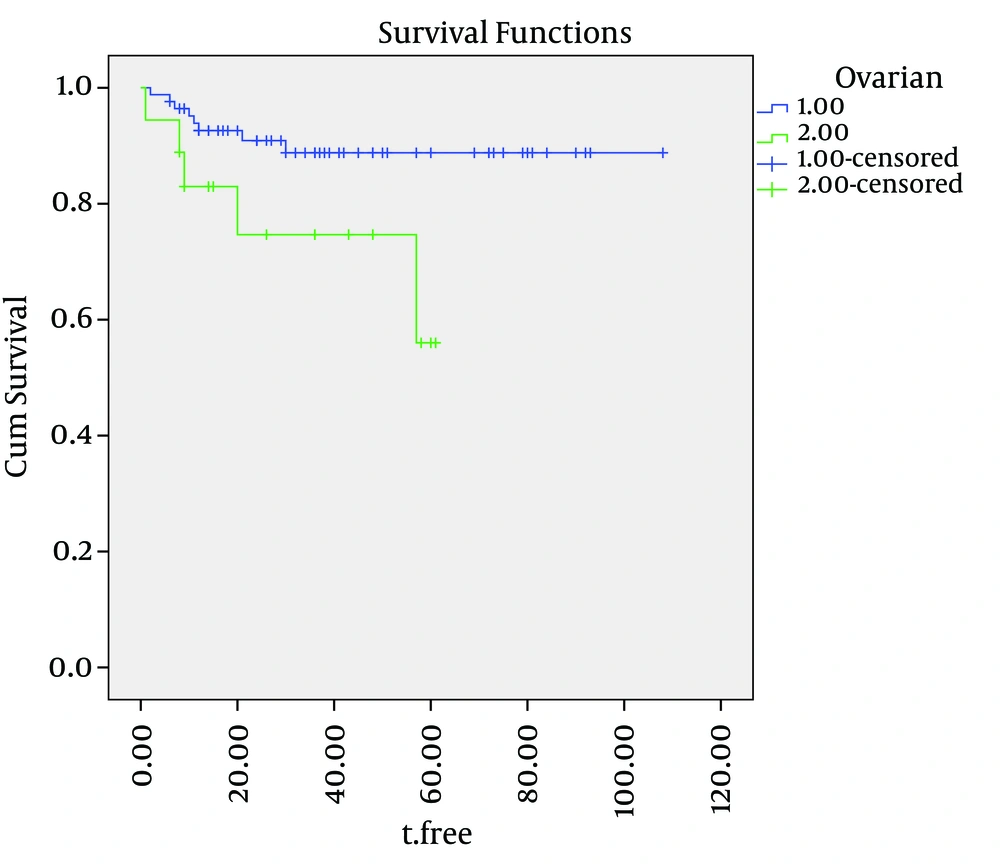
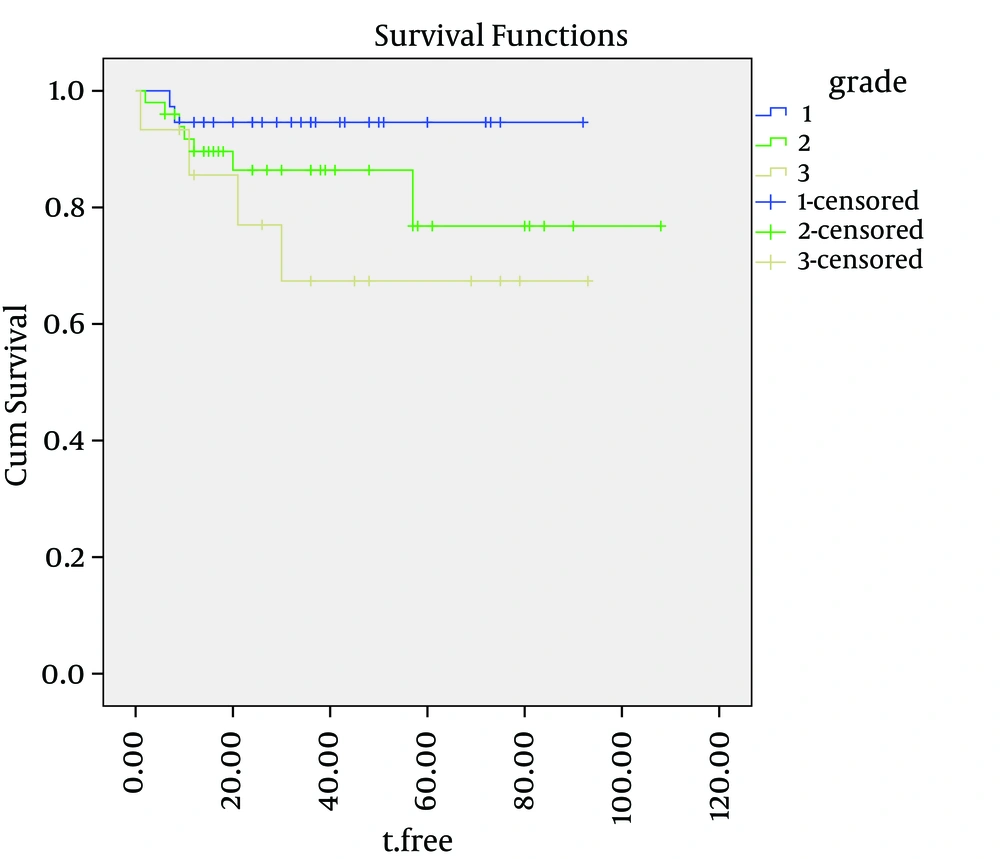
Cause-specific survival rates of the entire group were 92%, 87% and 82%, at two, three and five years, respectively. The five-year cause-specific survival rates for grades one, two and three were 98%, 88% and 68%, respectively. The cause-specific survival curves for the depth of myometrial invasion, ovarian involvement and tumor grade are shown in Figures 1, 2 and 3. depth of myometrial invasion, involvement of the ovaries, tumor grades and involvement of serosa were independent predicators for cause specific survival (log-rank = 0.001, 0.015, 0.024, and 0.030, respectively). However, lymph-vascular space invasion (log-rank = 7.1) and cervical invasion (log-rank = 0.089) did not correlate with DFS.
No significant association was found between survival and lymph node states (P = 0.311).
At the time of analysis, 20 (9.8%) of the patients had recurrence: four local, twelve distant, and four both local and distant. Four patients with only local failure were successively treated, yet all the sixteen patients who had a distant component of relapse died within the same year. All recurrences occurred at a mean time of 20 months. There were eight (3.9%) post-operative complications: four wound infections and four ileus episodes requiring a prolonged hospital stay. No surgical mortality was seen, and no patient developed a major complication directly related to the lymphadenectomy.
5. Discussion
Endometrial cancer is surgically staged and many of the known risk factors for OS, such as depth of myoinvasion, cervical invasion, adnexal metastasis, and lymph node metastasis, are captured by The International Federation of Gynecology and Obstetrics (FIGO) staging systems. However, other important risk factors that may affect OS are not included in the FIGO system. These factors are likely to include age at diagnosis, FIGO grade, histological subtype, and the adequacy of surgical staging. Overall survival is commonly reported in the endometrial cancer literature.
Regional spread of disease was found, despite low-risk pathological findings on a preoperative biopsy, and absence of myometrial invasion. This further highlights the importance of surgical staging of endometrial cancer, regardless of preoperative or intraoperative pathologic assessment, unless there are surgical contraindications. Other groups have found similar discrepancies between preoperative and final pathologic findings (4-6). Furthermore, Roland et al. (7) reported that surgical staging in patients with grade 1 endometrial cancer significantly impacted postoperative treatment decisions in 29% of patients. Fortunately, the patients with endometrial cancer are usually diagnosed at an early stage, thus surgical staging including lymphadenectomy derives excellent survival rates (8).
It is important to highlight that assigning patients to the correct FIGO stage requires surgical staging with node dissection, and although the therapeutic role of lymphadenectomy is debated, the value of lymphadenectomy in correctly assigning patients to the final surgical stage is much less controversial (9).
A variety of risk factors for recurrence have been identified in different studies, with discrepancies in risk factors likely due to the small numbers of patients available for retrospective review in most studies (10-12).
This article demonstrates that there is an age-specific decrease in survival for patients diagnosed with endometrioid adenocarcinoma of the uterus. Specifically, after the age of 50, this survival decreases below 80%. Previous articles have described a relationship between age and survival with respect to endometrial cancer (13-15).
Although stage of disease is the most significant prognostic variable, a number of factors have been shown to correlate with outcomes in patients with the same stage of disease. Knowledge about these factors is essential if appropriate treatment programs are to be advised. The degree of histologic differentiation of endometrial cancer has long been accepted as one of the most sensitive indicators of prognosis. As the tumor loses its differentiation, the chance of survival decreases. Most patients present early-stage, low-grade (grade 1) disease and experience five-year survival rates approaching 80 - 90% (16-18). However, a subgroup of patients will present grade 3 of the disease, a distinct, more biologically aggressive endometrioid subtype which has a higher risk of both locoregional and distant relapse and poorer survival outcomes. There are several studies suggesting that histological grade is one of the most important prognostic factors for recurrence and outcomes in patients with early-stage endometrial cancer. These studies cumulatively demonstrate that patients with early-stage, grade three endometrioid adenocarcinoma of the endometrium have poorer outcomes than those with early-stage, lower grade of the disease. In their review of 244 patients with stage one disease, Genest et al. noted that patients with grade one had a survival of 96%, this dropped to 79% and 70% for grade two and grade three, respectively (19). Fujimoto et al. demonstrated that patients with stage I - III, grade three disease experienced higher locoregional recurrence rates than patients with lower grade, similarly staged disease (20). Grigsby et al. reported five-year progression-free survival rates for grade three tumors with superficial and deep myometrial invasion of 69% and 42%, respectively, compared with 70% to 95% for the other stage I subgroups (21). Furthermore, Creutzberg et al. confirmed that grade three-endometrioid histology was one of the most adverse prognostic factors for recurrence, with an HR of 5.4 (22).
Our findings did not reveal an association between survival and lymphadenectomy with preoperative early stage endometrioid type endometrial cancer. Comprehensive surgical staging for patients with endometrial cancer remains controversial and ranges from universal lymphadenectomy (23) to lymphatic assessment in only those with adverse risk factors (24).
Vascular space invasion appears to be an independent risk factor for recurrence and for death from endometrial carcinoma of all histological types (25). Aalders et al. reported recurrences and deaths in 26.7% of patients with stage one disease, who had vascular space invasion, compared with 9.1% of those without vessel invasion (17).
Abeler et al. reviewed 1974 cases of endometrial carcinoma from the Norwegian Radium Hospital and reported an 83.5% five-year survival rate for patients without demonstrable vascular invasion compared with 64.5% for those in whom invasion was present (26).
Ambros and Kurman, using multivariate analysis, reported that only depth of myometrial invasion, DNA ploidy, and vascular-invasion-associated changes correlated significantly with survival for patients with stage one-endometrioid adenocarcinomas (27).
The majority of recurrences were diagnosed within the first two years after surgery (28). In our series, initial recurrence occurred after a median of 20 months, which was consistent with many other series.
We understand the weaknesses of this study including its retrospective nature and lack of central pathology review. The retrospective nature does introduce potential selection bias; however, it should statistically affect all patients equally. Although there was a lack of 100% central pathologic review, all surgeries were performed at one institution in which a pathologist specializing in gynecologic pathology reviewed all pathologies at the time of diagnosis and at a gynecologic oncology conference.
In conclusion, regional spread can occur in endometrial cancer, despite low risk pathologic findings on initial biopsy. Recurrences occur during all stages of initial disease in endometrial cancer and are uniformly associated with poor survival. Local recurrence rates are low after surgery and adjuvant radiation therapy. Several pathologic and treatment-related factors should be considered when choosing an adjuvant treatment regimen. In our study, age, high grade, deep myometrial invasion, ovarian and serosa involvement were independent predicators for cause specific survival. However, lymph-vascular space invasion and cervical invasion did not correlate with DFS. The results of this calculation may help clinicians offer better patient counseling on clinical outcomes and provide more individualized planning of postoperative management.