1. Background
The main renal function is to excrete creatinin and potassium, while preserving sodium and water. This normal renal task can be disturbed when there is an obstuction in the urinary tract, which is called obstructive uropathy (OU). OU is a rare, but curable, cause of acute renal failure; however, after releasing a long lasting obstruction, the kidney response to the volume and the toxin overload may become destructive per se. This state leads to excretion of a huge amouts of water and sodium and is called pathological postobstructive diuresis, which can cause severe hyponatremia and death. Taking this issue into consideration, measuring urinary output as the main indicator of this phenomen is outmost important, and several protocols are introduced to overcome this renal malfunction.
During OU, renal blood flow and glomerular filtration rate (GFR) may decrease, causing tubular abnormalities such as urinary concentration defect (1). Moreover, expression of aquaporin is downregulated after long lasting bilateral ureteral obstruction (BUO), suggesting a contribution to long-term polyuria and impairment of urinary concentrating capacity that happen after relief of OU (2-10).
It is reported that sildenafil citrate, a phosphodiesterase type 5 inhibitor (PDE5 inhibitor), restores the downregulated aquaporin channels in kidney caused by OU. This may theoretically improve renal function in such patients. The current study aimed at assessing the effects of sildenafil citrate on renal function after BUO release.
2. Methods
A total of 28 male adult Munich-Wistar rats, weighing 200 - 250 g, were enrolled in the current study. The surgery-induced BUO was performed on all rats and after 24 hours BUO was released. In the sildenafil group (n = 14), sildenafil citrate (200 mg/kg food) was given to the rats from the surgery day, while the control group had just standard rat food. Daily urinary volume, urine sodium, potassium, and osmolality were measured every 24 hours from the day before the surgery to the second postsurgical BUO releasing day.
2.1. Induction of BUO
The animals were anesthetized intraperitoneally with 50 mg/kg sodium thiopental. With a midline lower abdominal incision, ureters were removed. Two ligatures, 5 mm apart, were placed in the mid part of the ureter over a section of polyethylene tubing (PE-70) around each ureter. The ureters were occluded by tightening the tubing with a 3 - 0 silk ligature. After recovering from anesthesia, the animals were returned to their metabolic cages with free access to water and standard rat food based on the specific protocol for each group.
2.2. Collection of the Urine
To collect urine samples and measure daily urine production, metabolic cages were used. The daily urinary output (mL/day) was measured for each rat and urine samples were sent to the laboratory to be evaluated for osmolality and sodium/potassium measures.
2.3. Urine Osmolality
Urine osmolality was determined using a freezing point osmometer (Wescor 5520, USA) and expressed as mOsm/kg H2O.
2.4. Statistical Analysis
SPSS version 22 was used for the statistical analyses. The qualitative data were expressed as mean ± standard deviation (SD) and the quantitative data were shown in relative frequency. Based on the data volume (lower than 30) of each group, the Kolmogorov-Simonov test was used to examine the normal distribution of data. The nonparametric Wilcoxon signed rank test was used, before and after the BUO release, to evaluate the intergroup differences for the parametric variables, while nonparametric Mann-Whitney U test was used to compare intragroup differences for the same variables. P value < 0.05 was considered as the level of significance.
3. Results
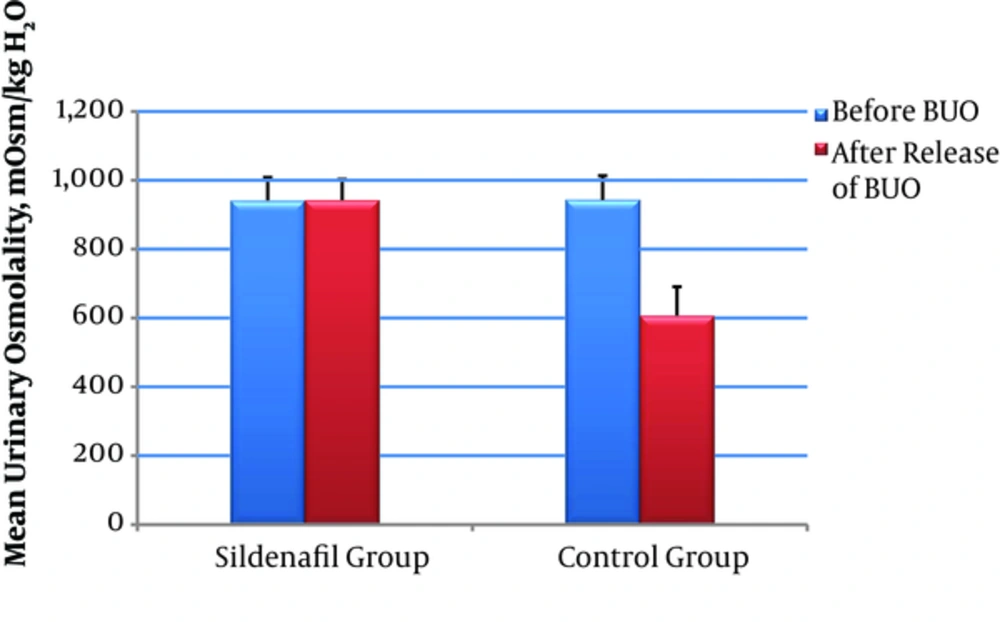
Changes in urinary parameters before and after BUO release in both sildenafil and control groups are presented in Table 1. There was an impaired urinary concentrating capacity 48 hours after the release of BUO in control group, compared with that of the Sildenafil group (P value < 0.001) (Figure 1).
| Urinary Parameters | Intervention (n = 14) | Control (n = 14) | P Value Intragroup | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before BUO | After the Release of BUO | Z-Statistic | Intergroup P Value | Before BUO | After the Release of BUO | Z-Statistic | Intergroup P Value | |||
| K, mEq/d | 2.14 ± 0.37 | 2.14 ± 0.22 | - 0.414 | 0.679 | 2.16 ± 0.4 | 2.27 ± 0.41 | - 2.068 | 0.059 | 0.285 | |
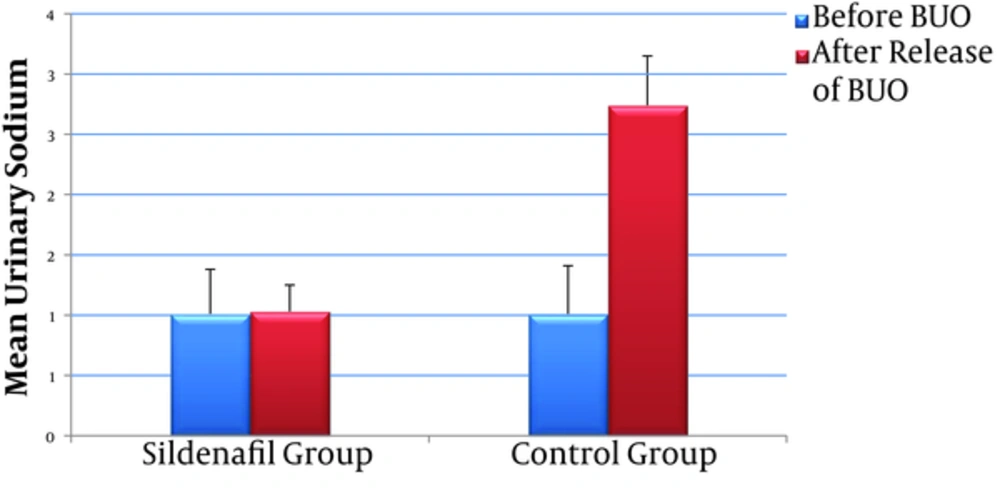
| Na, mEq/d | 1.01 ± 0.31 | 1.07 ± 0.23 | - 0.794 | 0.427 | 1.01 ± 0.31 | 2.74 ± 0.93 | - 3.301 | 0.001 | 0.001 | |
| Osmolality, mOsm/kg H2O | 941.50 ± 66.71 | 942.21 ± 60.94 | - 0.974 | 0.330 | 943.14 ± 70.09 | 606.64 ± 84.10 | - 3.296 | 0.001 | 0.001 | |
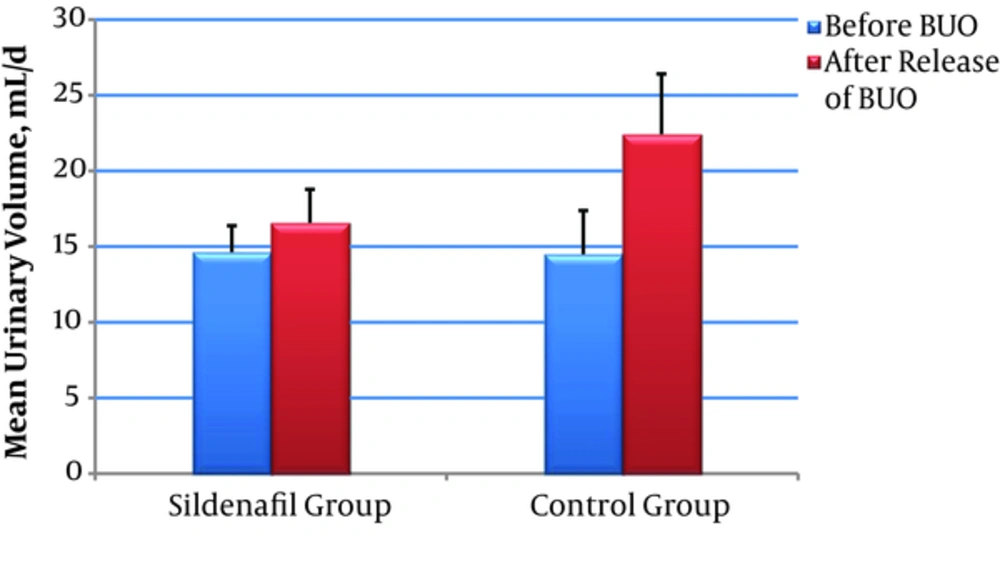
| Volume, mL/d | 14.64 ± 1.74 | 16.57 ± 2.21 | - 2.325 | 0.200 | 14.50 ± 0.4 | 22.43 ± 0.37 | - 3.306 | 0.001 | 0.001 | |
Rats were studied from 24 hour before BUO to 48 hours after releasing the obstruction; There was a significant increase in urinary Na concentration and daily urine volume in control group after releasing the BUO (P value < 0.001), While these parameters did not change significantly in sildenafil group (P value = 0.427 and 0.200 respectively) (Figures 2 and 3).
4. Discussion
Obstructive uropathy is a condition with many different physiological and pathological consequences that can cause transient or permanent functional disability to kidneys. This impairment in some degrees depends on the duration of obstruction (acute or chronic) (11, 12).
In OU, the initial event is vasodilatation, which lasts 90 minutes; then, more prominent drops occur in renal blood flow, that maybe attributed to release of strong vasoconstrictors, angiotensin II, and thromboxane A2 (7, 11, 13). During this period, intraurethral pressure is above normal range and intrarenal blood-flow is redistributed from medullar to cortex (13). These events under BUO conditions can increase renal vascular resistance and then, reduce GFR (14, 15).
Following the treatment of OU, some physiologic changes, such as intravascular expansion and subsequently atrial natriuretic peptide (ANP) secretion, occur that finally leading to efferent and afferent arteriolar vasodilatation. After these changes, with increasing glomerular capillary pressure and reducing renin production, an absolute increase occurs in GFR, which as a clinical finding, is referred to postobstructive diuresis. As expected, in unilateral ureteral obstruction elimination of harmful vasoactive agents by contralateral kidney through secretion of ANP attenuates or even prevents these harmful changes (7, 11, 15).
In association with postobstructive diuresis, some electrolyte changes are occurred from which the most important ones are the alteration in urinary excretion of sodium and potassium, and changes in urinary osmolality. As expected, in patients with unilateral ureteral obstruction and normal contralateral kidney, compensation mechanism can overcome, though partially, and reabsorb water and electrolytes (12, 16).
Under normal conditions, any changes in serum osmolality or effective circulating volume may directly affect the secretion of vasopressin from posterior hypophysis. The vasopressin is an antidiuretic agent that acts on the receptors of basolateral membrane of kidney collecting duct cells, which causes the fusion of vesicles containing aquapurins into apical cells of the collecting ducts. As a consequence, an impermeable membrane converts into a water-permeable membrane, through the action of aquapurins AQP2, AQP3, and AQP4 (8-10).
In patients with BUO caused by ischemia, vasopressin hyposensitivity, and downregulation of aquaporin receptors, the low osmolality urine with increased sodium concentration was excreted, which explains long-term polyuria and urinary concentrating defects (2-10, 17).
The current study showed the effects of sildenafil in a BUO model. The results of the current study demonstrated that sildenafil administration prevented postobstructive diuresis 48 hours after the release of a 24-hour BUO. Treatment with sildenafil prevented BUO-related hemodynamic effects, such as decreased urine osmolality and polyuria. In sildenafil-treated rats, urinary osmolality was better saved than that of the control group. These deleterious effects could be reversed by sildenafil administration and it may imply the clinical application of sildenafil in acute obstructive nephropathy. Further human models are required to better understand the reported mechanism.
This study had several limitations; for instance, the limited number of cases and lack of a sham group. Variables in the study were limited to some urinary parameters, without any serum parameters such as serum sodium and potassium. Maybe the most appropriate way to collect and report urine samples is to check urinary output hourly (rather than daily) in order to confirm pathological post-obstruction diuresis, but due to lower level of urinary output in rats (~0.5 - 0.75;mL/hour), it was very difficult to collect and measure urinary output hourly.
4.1. Conclusions
Sildenafil citrate significantly improved urinary concentration by reversing the BUO-induced downregulation of AQP2 in a rat model. These findings may have significant clinical implication in treating obstructive uropathy.