1. Background
The outbreak of overweight and obesity among adolescents is a global challenge (1). Obesity-induced systemic low-grade inflammation is characterized by the alterations of circulating concentrations of pro- and anti-inflammatory cytokines (2). Interleukin-10 (IL-10), a powerful anti-inflammatory peptide, plays a beneficial role in human metabolism (3) and insulin sensitivity (4). In the condition of obesity, the number of cells secreting interleukin-10 is decreased; consequently, resulting in the decreased circulating levels of IL-10. Such alterations in cytokine secretion are thought to cause a systemic low grade inflammation contributing to the increase of insulin resistance and metabolic syndrome (5). It has been proved that weight-loss exercise programs are accompanied by increased circulating levels of IL-10 in obese individuals; thereby creating improved metabolic disorders (3). Physical activity is recommended for the management of overweight among adolescents (6), and its anti-inflammatory effects on a regular basis are well demonstrated (7). Basically, aerobic workouts are used for overweight and obese adolescents (8-10); nonetheless, high-intensity interval training (HIIT) (11-14) and resistance training (RT) (15-19) also offer a number of physical health-related benefits such as reducing fatness, improved blood glucose, insulin sensitivity, lipid profile, and improved body composition, which are recommended for the weight management of overweight/obese population (14, 20). However, there is a paucity of information on the efficacy of both HIIT and CRT in inducing an anti-inflammatory cytokine response, and in improving metabolic health profile (lipid profile and HOMA-IR) and body composition in overweight male adolescents. Hence, this study was designed to evaluate the anti-inflammatory cytokine response, and to evaluate the metabolic profile and body composition in overweight male adolescents subjected to HIIT and CRT.
2. Methods
2.1. Study Design
Twenty overweight sedentary male adolescents participated voluntarily in this trial. All of the participants were high school students with the 17-19 age range and the body mass index (BMI) range of 25-30 kg/m2. Subjects were equally and randomly assigned into two groups of HIIT (N = 10) and CRT (N = 10). The percentage of the body fat was also calculated using Caliper (Lafayette made in America) and Jackson and Pollack’s three points formula (21) by measuring the subcutaneous fat of three muscles (triceps brachii, abdomen, and thigh). Body weight, BMI, and body fat percent of each group at baseline are depicted in Table 1.
| Exercises | %1RM | Numbers of Repetitions | Length of Each Exercise, s |
|---|---|---|---|
| Chest press (free weights) | 60 | 8 - 12 | 30 |
| Leg press (machine) | 60 | 8 - 12 | 30 |
| Shoulder press (free weights) | 60 | 8 - 12 | 30 |
| Seated rows (machine) | 60 | 8 - 12 | 30 |
| Leg extension (machine) | 60 | 8 - 12 | 30 |
| Triceps extension (machine) | 60 | 8 - 12 | 30 |
| Leg curl (machine) | 60 | 8 - 12 | 30 |
| Biceps curl (machine) | 60 | 8 - 12 | 30 |
aRest length between each exercise: 30 seconds. Rest length between each circuit: 120 seconds.
bPredicted 1-RM= weight lifted/[1.0278 - 0.0278(X)], Where X = the number of repetitions performed.
2.2. HIIT Protocol
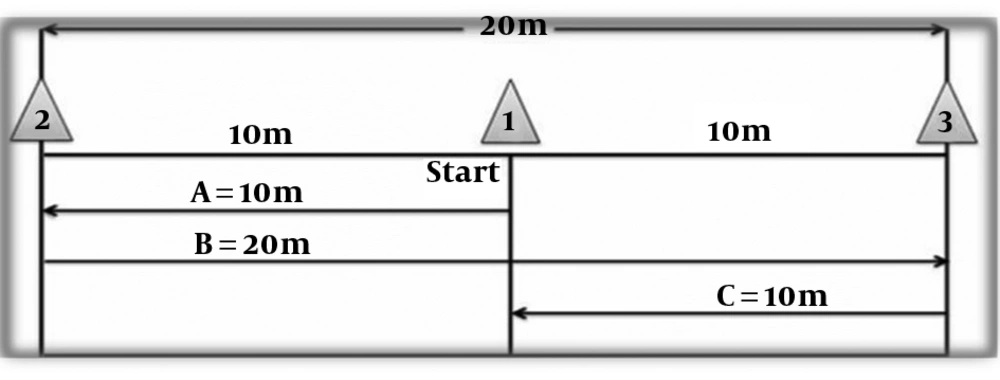
Participants in the HIIT group performed a sprint interval training (SIT) three times a week for six weeks. This protocol, also known as a shuttle-run test, was carried out in a gym. Each participant performed a 20-meters sprint for 30 seconds (Figure 1). Three cones were placed 10 meters apart. Each participant was required to sprint from the middle cone to the first cone, turning and sprinting in the reverse direction to the second cone, turning and sprinting again through the middle cone. After 30 seconds rest, the participants repeated this method for other 3 times. The overload principle was applied by increasing the number of repeats from four frequencies during weeks 1 and 2 to 5 frequencies during weeks 3 and 4 to 6 frequencies during weeks 5 and 6. Heart rate (HR) response was measured in all participants after completing each repetition via the use of heart rate monitor watch (Beurer, PM80, Germany) with a belt connected to the subject’s chest. Throughout the training protocol, the HIIT intensity was considered above 90% of peak heart rate and calculated by Karvonen formula. The peak heart rate was calculated by the equation, HRmax= 207 - 0.7 × age (y).
2.3. CRT Protocol
Participants in the RT group performed nine different exercises targeting the principal muscles. The exercises consisted of chest press, leg press, shoulder press, seated rows, leg extension, triceps extension, leg curl, biceps curl, and sit-up (Table 1). Each exercise included 8 - 12 frequencies at the workload of 60% of the subject's one repetition maximum (1 RM). The subjects underwent training for 6 weeks, 3 sessions per week. Each session consisted of three circuits and in each circuit 9 movements, previously mentioned were done one after another. The duration of each movement was 30 seconds (8 - 12 repeats), the duration of resting between two consecutive movements was 30 seconds, and the duration of resting between the two circuits was considered to be 120 seconds. Each session took a total time of 50 - 55 minutes. The principle of overload was designed after each four weeks of training. In this study, the Brzycki formula was used to calculate the 1RM (22).
Blood samples were drawn from all participants one day before the start of protocols and one day after the last session of the exercise protocols. The volume of each blood sample was 10 mL of the whole blood that was centrifuged at 3,000 rpm for 8 minutes at 4°C to separate the serum, and then stored at -80°C for future analysis. Analysis of IL-10 was performed by Enzyme immunoassay ELISA (enzyme-linked immunosorbent assay). The analysis of IL-10 (Cat No. 950.060.096, Diaclone, France) concentrations was performed using the kits purchased from Diaclone Company with the minimum detectable concentration of 4.9 pg/mL. The intra-assay and inter-assay variations were 3.5% and 7.5%, respectively. Analysis of insulin concentrations was performed by ELISA kit (Monobind Inc., Lake Forest, CA, USA) with a sensitivity of 0.75 µlU/mL, and intra-assay variability was 4.3% to 8.3%. Serum concentrations of total cholesterol (TC) high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), and glucose were measured by photometric method (Pars Azmun Company Kits, Iran). Insulin resistance index (IRI) was calculated using the homeostasis model assessment (HOMA). IRI = (fasting insulin [µU/mL] × fasting glucose [mmol/L])/22.5 (23).
The normality of the data was assessed by the Kolmogorov-Smirnov test. The paired t-test was applied for determining the possible changes in each group after 6 weeks of exercise interventions. Moreover, the independent t-test was used to verify possible differences between pre-training, as well as the magnitude of the variations (Δ) after training among the two groups. The SPSS version
16.0 was used to analyze the data and the significance level was considered < 5%.
3. Results
The pre-training and post-training values of the body weight, BMI, and body fat percent for each group are presented in Table 2. The HIIT significantly reduced body mass, BMI, and body fat percent of the participants; however, the RT increased the body weight, BMI, while reduced body fat percent insignificantly. The changes of the body weight and BMI were only significant between the groups. Serum concentrations of IL-10, lipid profile, insulin, and glucose are presented in Table 3. Serum concentrations of IL-10 were insignificantly increased in both groups; however, the concentration of IL-10 showed a more tendency to increase after HIIT (P = 0.060) than CRT (P = 0.60). There was no significant difference in the serum concentrations of IL-10 between the groups. The HIIT protocol significantly increased the serum concentration of HDL-C and decreased significantly serum concentrations of LDL-C, total cholesterol (TC), triglyceride (TG) in contrast to the RT protocol that had no effect on lipid profile of adolescent overweight boys. There were significant differences in terms of HDL-C, TC, and TC between the groups. The HIIT group, in contrast to the RT group, promoted significant changes in insulin resistance index in which the fasting serum concentration of glucose and insulin were significantly decreased. Furthermore, there were significant differences in terms of serum concentration of glucose and homeostasis model assessment of insulin resistance (HOMA-IR) between the groups.
| Variables/Group | Pre-Test | Pre-Test | ∆b | P Valuec |
|---|---|---|---|---|
| BW, kg | ||||
| CRT | 82.27 ± 4.06 | 83.10 ± 4.14 | 0.83 ± 0.25 | 0.071 |
| HIIT | 82.70 ± 3.6 | 80.40 ± 3.6d | -2.3 ± 0.4e | 0.001 |
| P valuef | 0.418 | 0.659 | 0.001 | - |
| BMI, kg/m2 | ||||
| CRT | 27.89 ± 0.73 | 28.56 ± 0.72 | 0.67 ± 0.1 | 0.117 |
| HIIT | 27.80 ± 1 | 26.50 ± 1d | -1.3 ± 0.1e | 0.000 |
| P valuef | 0.778 | 0.535 | 0.021 | - |
| BFP, % | ||||
| CRT | 22.56 ± 2.54 | 21.56 ± 2.53 | -1 ± 0.008 | 0.096 |
| HIIT | 22.5 ± 3 | 21.3 ± 3d | -1.2 ± 0.01 | 0.001 |
| P valuef | 0.987 | 0.977 | 0.516 | - |
Abbreviations: BFP, body fat percent; BMI, body mass index; BW, body weight; CRT, circuit resistance training (3 times a week, 6 weeks, 60% 1RM); HIIT, high-intensity interval training (3 times a week for 6 weeks, HRmax > 90%); SD, standard deviation.
aValues are expressed as mean ± SD.
bΔ, Post-test vs. pre-test values.
cPaired t-test.
dP < 0.05 compared pre- and post-test values (paired t-test).
eP < 0.05 compared the differences between the groups (independent t-test).
fIndependent t-test.
| Variables/Group | Pre-Test | Post-Test | ∆b | P Valuec |
|---|---|---|---|---|
| IL-10, pg/mL | ||||
| CRT | 5.8 ± 1.38 | 5.9 ± 1.79 | 0.1 ± 0.67 | 0.60 |
| HIIT | 5.04 ± 1.07 | 5.7 ± 1.26 | 0.7 ± 0.98 | 0.06 |
| P valued | 0.168 | 0.731 | 0.16 | |
| HDL, mg/dL | ||||
| CRT | 39.5 ± 5.5 | 41.3 ± 7.40 | 1.8 ± 4.78 | 0.264 |
| HIIT | 41.9 ± 5.91 | 48.9 ± 7.68e | 7 ± 4.05f | 0.000 |
| P valued | 0.360 | 0.037 | 0.017 | |
| LDL, mg/dL | ||||
| CRT | 122.2 ± 24.09 | 119.7 ± 24.48 | -2.5 ± 13.45 | 0.571 |
| HIIT | 140.7 ± 30.7 | 127.3 ± 27.33e | -13.4 ± 9.98 | 0.002 |
| P valued | 0.152 | 0.521 | 0.056 | |
| TC, mg/dL | ||||
| CRT | 163.2 ± 28.42 | 162.2 ± 31.45 | -1 ± 11.47 | 0.789 |
| HIIT | 172.4 ± 22.07 | 157.1 ± 19.95e | -15.30 ± 14.22f | 0.008 |
| P valued | 0.430 | 0.671 | 0.024 | |
| Glucose, mg/dL | ||||
| CRT | 87.10 ± 6.08 | 88.60 ± 6.38 | 1.5 ± 4.62 | 0.332 |
| HIIT | 89 ± 5.41 | 85.7 ± 6.63e | -3.3 ± 4.6f | 0.049 |
| P valued | 0.470 | 0.332 | 0.032 | |
| Insulin, µL U/mL | ||||
| CRT | 7.75 ± 4.63 | 7.76 ± 4.46 | 0.019 ± 0.90 | 0.948 |
| HIIT | 7.72 ± 4.0 | 6.96 ± 4.16e | -0.76 ± 0.96 | 0.034 |
| P valued | 0.989 | 0.681 | 0.079 | |
| HOMA-IR | ||||
| CRT | 1.64 ± 0.93 | 1.67 ± 0.91 | 0.033 ± 0.21 | 0.632 |
| HIIT | 1.68 ± 0.86 | 1.45 ± 0.86e | -0.22 ± 0.25f | 0.022 |
| P valued | 0.926 | 0.587 | 0.025 |
Abbreviations: CRT, circuit resistance training (3 times a week, 6 weeks, 60% 1RM); HIIT, high-intensity interval training (3 times a week for 6 weeks, HRmax > 90%); HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; IL, interleukin; LDL, low-density lipoprotein; SD, standard deviation; TC, total cholesterol; TG, triglyceride.
aValues are expressed as mean ± SD.
bΔ, Pre-test vs. post-test values.
cPaired t-test.
dIndependent t-test.
eP < 0.05 compared pre- and post-test values (paired t-test).
fP < 0.05 compared the differences between groups (independent t-test).
4. Discussion
Our study demonstrated that (a) HIIT had more effect on inducing an anti-inflammatory cytokine response via increasing the basal serum levels of IL-10 to a greater extent than CRT, and (b) HIIT is superior to CRT in improving body composition, lipid profile and insulin resistance index in overweight male adolescents. The present investigation failed to find a significant change in resting circulating level of IL-10 following 6 weeks of HIIT in overweight male adolescents. Two studies of Leggate et al. (24) and Kelly et al. (25) showed that a 2-week HIIT did not change the circulating levels of IL-10 in the overweight/obese males. Moreover, Zwetsloot et al. (26) showed that two weeks of HIIT did not make any difference in the resting circulating levels of IL-10 in recreationally active young males. However, we observed a considerable increase in the circulating level of IL-10 (+0.4 ± 0.87Δ, P = 0.060) in response to HIIT after six weeks of training in overweight adolescents.
The reasons for these discrepancies can be due to the type, duration and intensity of HIIT protocols, and also the subjects involved in the trials. The studies addressing the anti-inflammatory response to HIIT in overweight/obese population is scarce that warrants more researches should be carried out to understand the mechanisms by which HIIT stimulates an anti-inflammatory response in the overweight/obese population. Similar to the HIIT group, six weeks of CRT did not change the resting serum level of IL-10 in overweight male adolescents with the difference that HIIT produced more considerable changes in the resting serum level of IL-10 in overweight male adolescents. A study using a 10-week RT protocol showed no change in the resting circulating level of IL-10 in sedentary females (27). However, it is concluded that 12 weeks of RT improves resting circulatory levels of IL-10 in obese women (28). Two studies have claimed that exercise intensity has a profound effect on the inflammatory response, resulting in higher anti-inflammatory cytokine responses (29, 30). However, the intensity of workloads in our protocol was considered moderate and fixed. Therefore, it is likely that the intensity used in our protocol is one of the determining factors for not inducing an anti-inflammatory response in the overweight adolescent cohort. Another variable that should be considered is the duration of the protocol. A longer period of training would probably allow further physiological adaptations that might show more robust effects on cytokine responses. The beneficial anti-inflammatory effect of RT has not been consistent between all studies. The mixed findings of the effects of RT could be due to a large number of utilized training protocols (31-33). The mode, intensity, and duration of exercise can be modified and all these factors have been shown to affect the response in terms of inflammatory markers (32). We also observed an improved body composition, lipid profile, and insulin resistance index (HOMA-1IR) in response to HIIT in overweight male adolescents. Contrary to the present findings, the results of a meta-analysis study (34) suggest that short term HIIT (< 12 weeks) has no effect on the body weight, percent of body fat, fasting insulin, lipid profile, and fasting insulin, but reduces fasting glucose by a small effect on overweight/obese populations. The reasons for this contradiction may be due to the baseline activity level (recreationally active), age conditions (≥ 18 years) and gender (female) of the overweight population investigated in the aforementioned meta-analysis study, whereas the subjects participating in our study were sedentary overweight adolescents ranging from 16 to 19 years. However, there are some studies showing the efficacy of the HIIT among the protocols in improving the body composition (10, 35, 36), lipid profile (13, 36, 37), and HOMA-IR (10, 38, 39) in healthy, overweight, and obese adolescents (36, 37) included 30-s sprints interspersed with 30-s rest bouts are of effective protocols to improve metabolic profile. Further studies are needed to be done to support these findings in overweight male adolescents. Contrarily, 6 weeks of resistance training did not improve the body composition, lipids profile, and insulin resistance condition in overweight male adolescents. There are few trials with conflicting results examining the effects of resistance exercise training on the body composition in overweight and obese adolescents (18, 40-43). Some studies have shown decreases (18), whereas others reported no difference (40) or increases (41) in the body fat percent of obese adolescents following a resistance exercise training program. Shaibi et al. study (18) showed that resistance training decreased significantly the body fat percentage of male adolescents. However, Davis et al. study (41) revealed no change in the body composition after 16 weeks of strength training. Benson et al. observed that 8 weeks of high-intensity resistance exercise training induced useful results in overweight/obese adolescents (43). When designing the resistance training, the intensity of a workout is essential as the higher intensity of resistance exercise training would also be beneficial in overweight/obese adolescents (44). The intensity applied to our resistance protocol was considered fix and moderate (60% 1RM), which may be one of the factors contributing to the achieved results. Besides the exercise intensity, the protocol duration (6 weeks) and the small numbers of subjects (n = 10) could be the other factors affecting the achieved results. Another variable analyzed was HOMA-IR. Our study showed that 6 weeks of circuit resistance training did not decrease fasting serum glucose and insulin in overweight male adolescents. A meta-analysis study emphasizes the efficacy of exercise in reducing fasting insulin and improving insulin resistance in adolescents (45). Moreover, van der Heijden et al. found that 12 weeks of resistance training in obese Hispanic adolescents induced an increase in hepatic insulin sensitivity (9). Shaibi et al. study (18) showed that 16 weeks of resistance training significantly increased insulin sensitivity in overweight or obese male adolescents. Another variable analyzed was lipid profile. In this study, a 6-weeks resistance training program did not improve lipid profile in sedentary overweight male adolescents. Results of other studies indicate that regular exercise decreases lipid profile (46, 47). However, some studies did not find any changes in lipids within overweight/obese adolescents (42, 43, 48). Altogether, six weeks of moderate intensity resistance training had no effect on anthropometric parameters and metabolic health in overweight male adolescents.
4.1. Conclusions
In conclusion, it seems that HIIT has more potent impact than CRT on inducing an anti-inflammatory cytokine response. Moreover, HIIT is a more appropriate exercise modality than CRT to improve body composition and metabolic profile (lipid profile and HOMA-IR) in overweight male adolescents. More studies are needed to support these findings in overweight adolescents' cohort.