1. Background
Low physical activity and consuming a high-calorie diet can induce obesity, which has become a major world health problem. It has been reported that some diseases such as motor disorders, cognitive disorders, cancer, metabolic syndrome, and cardiovascular disease are closely linked to obesity (1). Metabolic syndrome, which has a significant effect on cardiovascular disease-induced incidences of death, consists of some metabolic disorders, such as hypertension, obesity, lipid disorders, and increased insulin resistance (2). According to many studies, the prevalence of this syndrome is high in both Western and Asian countries. Controlling risk factors in people with metabolic syndrome can reduce the risk of this syndrome as well as other chronic diseases, such as cardiovascular disease and diabetes (3). More sensitive measurements of contractile function (e.g., left ventricular shortening fraction, systolic velocity, and myocardial pressure) are impaired in obesity and metabolic syndrome. The first step to reducing the risk factors is lifestyle modification (4), in which case Physical activity can be very effective. One of the most beneficial training methods to consider is high intensity interval training (HIIT). The intensity of HIIT is approximately between 80-100 percent of maximum oxygen consumption (VO2max) or maximal heart rate (HRmax). This method stands out for its very low duration, and as various studies have reported, it can improve aerobic performance. In this regard, researchers have conducted extensive studies in response to the question of what mechanisms in HIIT can improve aerobic performance (5). The results showed that peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is the most valuable marker of mitochondrial biogenesis with HIIT (5). Therefore, the researchers attributed aerobic performance improvement to mitochondrial biogenesis. PGC-1α is a cellular receptor belonging to a relatively large family of nuclear receptors associated with a wide variety of transcription factors in biological responses (6). Members of the PGC-1α family are activated in response to the environmental stimuli (e.g., heat and physical activity) and play an important role in maintaining energy homeostasis, lipid and glucose, and possibly in other conditions, such as neurodegeneration, diabetes, obesity, and heart disease (7). HIIT enhances PGC-1α gene expression and increases the mitochondrial gene expression of respiratory chain proteins of the mitochondria (8). Endothelial nitric oxide synthase (eNOS) is also one of the most important candidate genes in the prediction of coronary artery disease. eNOS is expressed by the NOS3 subunit, located on chromosome 7, and its length is approximately 21 kb (9). Continuous production of this enzyme maintains the vascular volume and function (10). HIIT restores NO concentration and its metabolites to the normal state and reduces vascular abnormalities. It also increases eNOS mRNA in the heart muscle, energy efficiency, physical function, and cardiac protection (11).
2. Objectives
For decades, aerobic training has been used to improve obesity, which is performed continuously through a specific activity over a specified time.
The features of these trainings consistently work for a relatively long period of time and devote between 150 and 250 minutes per week to achieving the goals. This kind of training causes people to be boredom after a while (due to the uniformity of the training process); therefor, continuous training programs cannot be a beneficial training in many cases.
However, HIIT is a type of interval training in which the intensity of activity is high, the time between intervals is low, and the dominant energy system is the anaerobic system. Recent studies on HIIT indicate that this method can be capable of producing physiological changes in different body systems, sports performances, and relevant markers of metabolic diseases. It can also be even more effective than traditional aerobic training. Although the effect of exercise with different training protocols on obesity-related factors has already been reviewed, most of the reported studies are endurance and continuous trainings, so there is little research on the effect of HIIT on eNOS and PGC-1α gene expression in obese and metabolic syndrome subjects. The present study aimed to investigate the effect of six weeks of HIIT on cardiac gene expression levels of eNOS and PGC-1α in male obese rats.
3. Methods
In this experimental study, 21 male Wistar rats with a mean age of 8 weeks selected as a sample and for adaptation to the laboratory environment kept one week at standard animal lab. Then 14 rats were given a standard high-fat diet (HFD) for two months.
In the present study, a high fat diet (containing 45% of the total energy from fat (derived from animal fat) comprising 24 g of fat (saturated fatty acid), 24 g of protein, and 41 g of carbohydrate per 100 g) was used for obesity induction (1). The obese rats were then placed into obese control and training groups. The remaining 7 rats were placed in the non-obese control group to review the effects of obesity on the study variables. During six weeks, rats in the training group performed HIIT three sessions per week. The training comprised 5 sets of alternation, 1 minute of training, 1 minute of rest on the treadmill at an intensity of 80% - 95% of Vo2max, and slow alternation at an intensity of 55% of Vo2max, reaching 10 sets of alternation in the last week. For warm-up and cooling down, rats ran for 5 minutes at an intensity of 40% of Vo2max before starting training and after finishing training (12). After six weeks, the rats were sacrificed, and their heart tissue was removed to measure the research variables. PGC-1α and eNOS gene expression levels were measured by real-time PCR. For molecular analysis at the gene expression level, RNA was extracted from the heart tissue by FavorPreTM Tissue Total RNA Mini Kit (FavorPrep, Taiwan), then the purity and concentration of RNA were evaluated by optical density measurements on a NanoDrop lite spectrophotometer (Thermo Fisher Scientific, USA). After extraction of high purity and high concentration RNA from all studied samples, cDNA synthesis was performed by the RevertAiTM First Strand cDNA Synthesis kit (Thermo Fisher Scientific, USA). Reverse transcription quantitative polymerase chain reaction (RT qPCR) was performed using the ABI Biosystems StepOne and the RealQ Plus 2× Master Mix Green (Ampliqon, Denmark). The Gap housekeeping gene was also used as an internal control of qPCR reactions. The qPCR conditions were set for 10 min at 94°C followed by 40 cycles of 15 sec at 94°C, 60 sec at 60°C, and final melt curve stage for product specificity analysis. The amplification signals of different samples were normalized to the Gap cycle threshold (Ct), and then livak method (2-ΔΔCt) was applied for comparing mRNA levels of different groups, which represented as fold change in data analysis. Table 1 shows the sequences of primers. We used the distribution of findings Shapiro-Wilk test to investigate the normality. We also employed one-way ANOVA with Tukey’s post-hoc tests to analyze the findings (P ≤ 0.05).
| Genes | Primer Sequences |
|---|---|
| B2m | Forward: CGTGCTTGCCATTCAGAAA |
| Reverse: ATATACATCGGTCTCGGTGG | |
| PGC-1α | Forward: GCACCAGAAAACAGCTCCAA |
| Reverse: TTGCCATCCCGTAGTTCACT | |
| eNOS | Forward: TGGCCAAAGTGACCATTGTG |
| Reverse: GGCAGGGGACAGGAAATAGT |
Sequence of Primers Used in the Study
4. Results
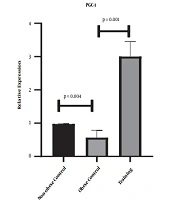
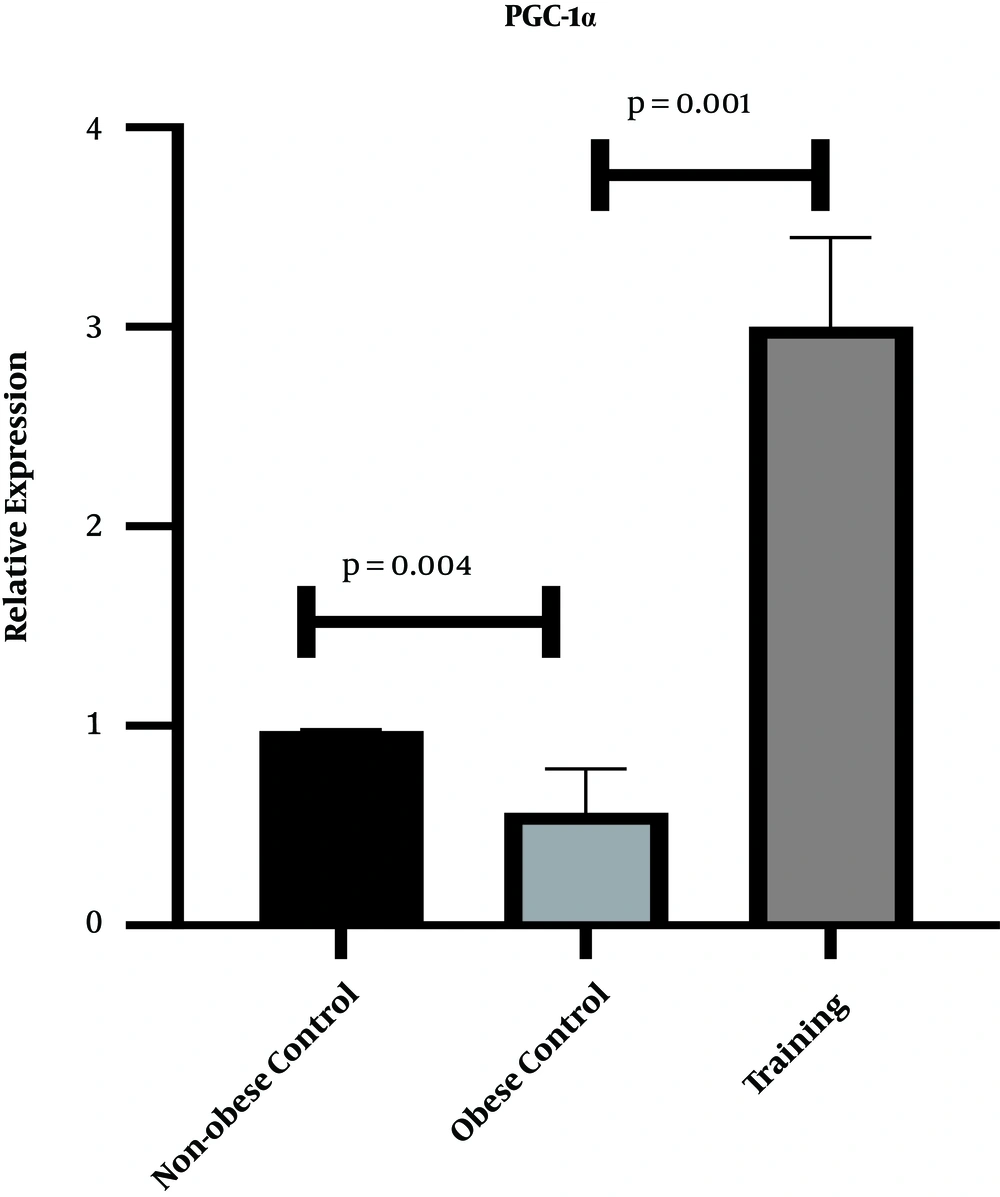
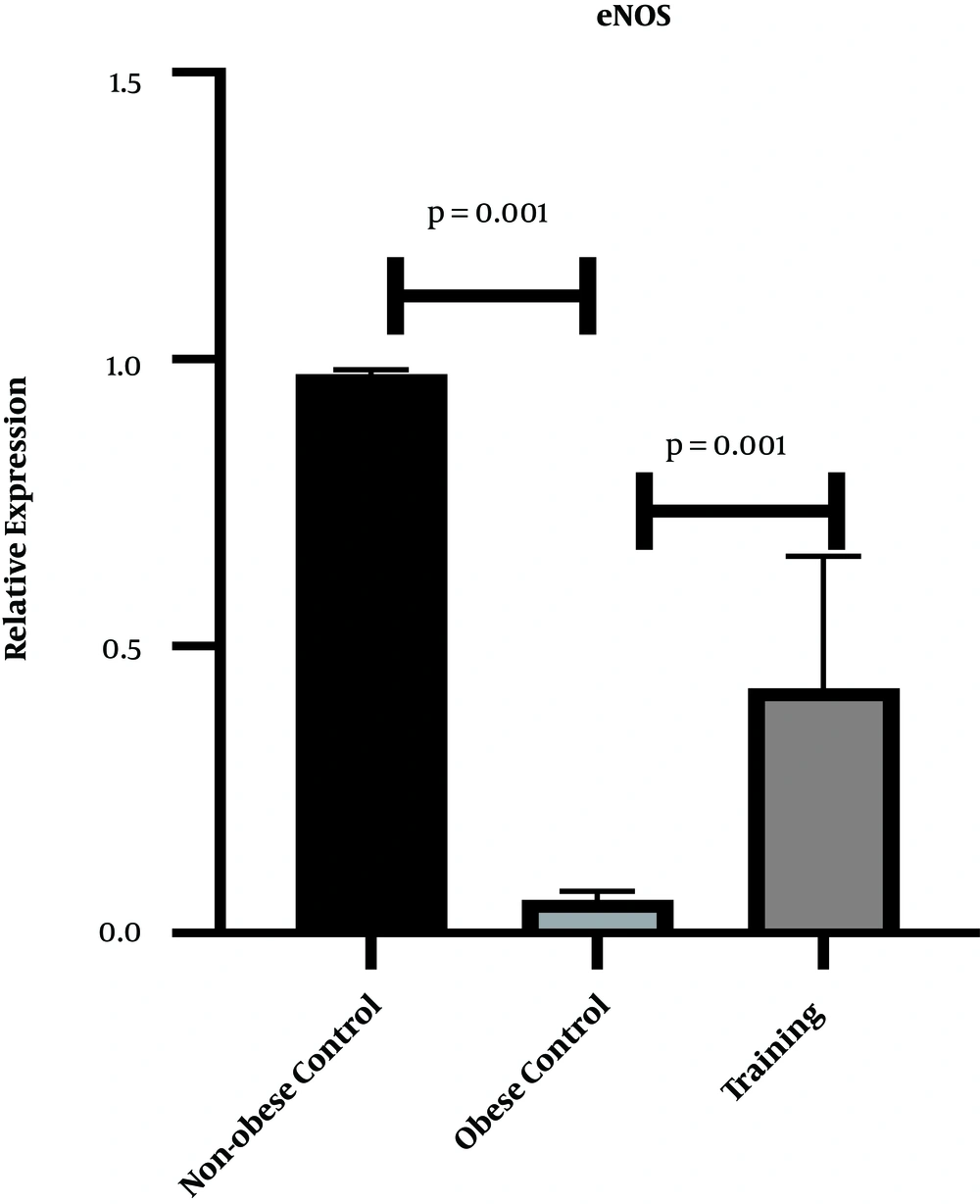
Figures 1 and 2 show the levels of PGC-1α and eNOS gene expression in the heart tissue of rats, respectively. The results of one-way ANOVA showed significant differences in cardiac gene expression levels of PGC-1α (P = 0.001, F = 144.63) and eNOS (P = 0.001, F = 84.39) in three research groups. The results of Tukey’s post- hoc test showed that the gene expression levels of PGC-1α (P = 0.04) and eNOS (P = 0.001) were significantly lower in the obese control group than the non-obese control group. However, the gene expression levels of PGC-1α (P = 0.001) and eNOS (P = 0.001) were significantly higher in the training group than the obese control group (Figures 1 and 2).
5. Discussion
In the present study, eight weeks of HFD regimen significantly decreased PGC-1α gene expression levels in the heart tissue of rats; nevertheless eight weeks of HIIT significantly increased eNOS and PGC-1α gene expression levels. As HIIT can enhance body composition, obesity-related diseases, and insulin resistance, HIIT became a popular training among society.
Cassidy et al. (13) reported several mechanisms in which HIIT may affect lipid profile. Lipid oxidation has been reported to increase during HIIT (13). Also, the levels of the enzymes and hormones involved in lipolysis increase (13). Consistent with the results of this research, Pugh et al. (14) showed that PGC-1α significantly increased after HIIT, which in turn led to skeletal muscle hypertrophy and reduced lipid (14). Also, 12 weeks of moderate-intensity continuous training (MICT) and HIIT had a significant effect on the PGC-1α protein content of obese rats, so that the effect of HIIT was significantly higher than MICT (15). They posited that these two types of training appear to increase mitochondrial biogenesis in subcutaneous fat, but the effects of HIIT are significantly greater (15). Unlike the findings of the present study, Chavanelle et al.’s (16) showed that none of the MICT and HIIT affected PGC-1α levels or other known regulators of mitochondrial biogenesis (16). It appears that exercising can increase mitochondrial biogenesis through the beta-adrenergic/ cAMP receptor pathway by increasing AMP / ATP ratio, AMPK, and up-regulation of PGC1-α/FNDC5/BDNF pathway, calcium concentration following physical activity activates calmodulin and AMPK-dependent enzymes, and sirtuin 1 (SIRT1). calcium release following muscle contraction activates calmodulin, calcineurin, and calmodulin kinase, resulting in increased SIRT1, PGC1-α, and peroxisome proliferator-activated receptors (PPAR) activation (17). Also, it has been reported that the expression of the PGC-1α gene following exercises results from the activity of phosphate- and calcium-dependent PGC-1α activation pathways, which activate adenosine monophosphate and calmodulin-dependent kinase enzymes that activate these pathways and ultimately activate them. High PGC1 expression can increase the mitochondrial content of the heart muscle and improve cell metabolism and reduces the production of free radicals. Therefore, it seems that the increase in PGC-1α after training can be physiologic in the present study.
Eight weeks of HFD regimen resulted in a significant decrease in cardiac gene expression of eNOS in male rats. In this regard, endurance training was shown to increase eNOS protein content in the heart tissue of rats, leading to angiogenesis in the left ventricle of rats; however, HIIT had no significant effect on eNOS elevation (18). In another study, Martensen et al. (19) demonstrated that in the moderate-intensity endurance training group, the mean endothelial thickness increased, and the basement membrane thickness decreased, whereas these structural parameters remained unchanged after HIIT. On the other hand, eNOS increased insignificantly in HIIT and in moderate intensity endurance training (19). It has been reported that exercise, especially interval exercise, can decrease oxidants and increase the antioxidants. Since superoxide oxidant reduces nitric oxide, the interval exercise improves nitric oxide bioactivity. Studies have also shown that insulin causes the release of nitric oxide, while insulin resistance impairs the release of nitric oxide (20). It has been noted that nitric oxide production can be attributed to a decrease in insulin resistance (20).
The present study encountered some limitations, such as lack of measurement of mitochondrial volume and mitochondrial enzymes. It is suggested that the mitochondrial volume and enzymes in the heart tissue be studied in the future. One of the weaknesses of this research was the absence of a non-obese training group. Thus, the non- obese training group should be used in the future along with the study groups of the present study. Also, considering that the type of training can be an important factor in the changes of PGC-1α and eNOS, it is recommended that future studies be regarding the effects of HIIT on negative and positive slopes.
5.1. Conclusions
It appears that HIIT plays an important role in increasing eNOS and PGC-1α gene expression in the heart tissue of male obese rats. However, further studies are required to confirm the present findings.