1. Background
Concomitant use of multiple drugs, also known as ‘polypharmacy’, is common in prescriptions made for critically ill patients hospitalized in the intensive care unit (ICU). Meanwhile, it is an essential part of ICU care (1, 2). The complexity of pharmacotherapies with different therapeutic classes of drugs (for the management of concurrent diseases) in one prescription and the possible administration-time overlaps, increase the risk of potential drug-drug interactions (PDI). PDI can be defined as “the possibility of altered therapeutic effects of one or more drugs due to simultaneous administration of several drugs to a biological system” (1, 3). The critically ill patients in ICU, with multiple severe diseases and/or organ failure (s), are at increased risk of polypharmacy-associated PDI, which may cause altered pharmacology of administered drugs, therapeutic outcomes of medications, the progression of the diseases, and, sometimes, detrimental physiological responses, known as ‘adverse drug reaction’ (ADR), extended hospital stay, and increased out-of-pocket expenditures (2, 4).
Most PDIs are preventable and, if be detected in the early stages of therapy, less harmful. Among the professionals involved in the ICU-pharmacotherapies, physicians are less aware of possible drug-drug interactions (DI), and sometimes critical care pharmacists (CCPs) mistakenly overlook such interactions, which lead to a higher risk of PDI (5-7). CCPs have a critical role in continuous monitoring of possible DIs and PDIs in the prescriptions because of their profound knowledge in the pharmacology of medicines, comprehensive medication management, and ADRs (8). Subscribed software-based drug-drug interaction checking system or incorporation of such facilities in the hospitals’ integrated system (HIS) are easy ways for CCPs to properly screen PDIs among the prescribed medications, and this system is less time consumable and less labor-intensive (9). In hospitals, where usually sufficient information technology-based resources are not available to ensure patients’ drug safety, HIS cannot provide such extended supports to CCPs for monitoring PDIs, and incorporation of online (with subscription-charge) drug-interaction checking software is not affordable; therefore hospitals mostly rely on CCPs’ skills and technical abilities to screen DIs/PDIs. Manual drug safety monitoring tasks are hard to read, time-consuming for CCPs. Besides, the possibility of unintentional PDI overlook among the prescribed medications is significant (9, 10).
Nowadays, smartphones are widely available, and health professionals are not an exception. As a result of this expansion of mobile health (mHealth), applications have become popular. Alert-making software can efficiently identify potential drug-drug interactions in prescriptions, and clinical pharmacists can ensure medication safety by utilizing these modern electronic tools (9).
2. Objectives
The current study aimed to evaluate the potential contributions of CCPs in monitoring the DIs/PDIs for ensuring medication safety among the prescriptions made for critically ill patients hospitalized in ICU of a resource-limited hospital-setup of Bangladesh by using subscription-free drug-interaction checker mobile apps.
3. Methods
3.1. Study Design
This two and a half year-long prospective, observational study was conducted in the ICU of Square hospital, a tertiary level private hospital in Dhaka (Bangladesh) from March 2016 to September 2018. During this time, in total, 3,041 patients were admitted in the ICU, and CCPs dealt with 2,967 patients (N). Hence data of 2,967 patients were analyzed in the current study. Every admitted patient in ICU has an individualized prescription profile. All prescriptions are electronically uploaded in the online patient-data archive of ‘Hospital Integrated System (HIS)’. All physicians and CCPs have access to current or previous patients’ prescriptions through HIS. All patient-wise prescriptions were analyzed by the CCPs for possible DIs/PDIs, and the ratio between patients and CCPs was 12:1. CCPs frequently report the patient-wise findings (DIs/PDIs) to the corresponding physicians, and prescriptions made for each patient review several times during the patient’s ICU-stay. Physicians frequently received notifications from CCP regarding their identified PDIs and modified the prescriptions, accordingly.
3.2. Process of Work and Data Collection
There was no online or offline DI checking system incorporated into the hospital’s HIS. CCP used the two most popular offline-based free DICMA on their mobile phones: ‘Medscape’ (by WebMD LLC.), a free DICMA; and ‘Epocrates’ (by Epocrates, Inc.). By using those free DICMA, CCPs were checking all prescribed drugs of all prescriptions of the admitted patients during the study time for DIs. Among those DIs, they identified the PDIs considering the patients’ disease status and medical co-morbidities and reported those PDIs to physicians to take corrective measures without delay. By using mobile phone-based apps, CCP could identify all PDIs spontaneously among the prescribed drugs within a very short time. The operating system of the DICMA was relatively similar and easy, and no specific training was required for CCP to handle the apps. CCP maintained a patient-wise ‘PDI recommendation form’ and after getting any PDI notification, they kept that record in that form as per the DICMA’s generated interaction-reports, and shared it with the physicians immediately for necessary actions. Data provided by CCPs’ were collected from ‘CCP suggestion forms’ (generated individual patient-wise) and the HIS data archive of the hospital. Demographic information of patients was collected from the patient history available in the HIS. To analyze the users’ (CCP) satisfaction level, a satisfaction scale was provided to the CCP who used the DICMA for checking drug interactions. Every CCP included in this study expressed his/her level of satisfaction in the context of user-friendliness and easy access to DICMA by pointing out a value in the satisfaction scale.
3.3. Sample Inclusion and Exclusion Criteria
Those patients that were admitted to the ICU and were received services from the CCPs were included in the study as participants. Admitted patients who did not use CCPs’ services (died or left the ICU before receiving CCPs’ interventions) were excluded from the study. No specific underlying condition or selection criterion was applied as inclusion or exclusion criteria. Therefore, only patients who left the ICU or died before getting any intervention from CCPs were excluded from the study.
3.4. Statistical Analysis and Ethical Approval
Ethical approval was taken in March 2017 from the ethical committee of the hospital. Pearson’s chi-square test was used to compare the categorical variables. Also, the student’s t-test was used for comparing continuous variables. Values were expressed with a 95% confidence interval (CI). A P value < 0.05 was considered as statistically significant. Data were analyzed with the Statistical Package for the Social Sciences (SPSS) version 22.0 statistical software (SPSS Inc., Chicago, United States of America).
4. Results
CCPs provided services to 2,967 critically ill patients in the ICU and identified 11,128 significant DIs in 2,782 patients (93.76%, n = 2,967) (P < 0.05) by using DICMA. Out of 2,782 prescriptions (with DIs), 2,226 (80.01%, n = 2,782) had PDIs (P < 0.05) (Table 1). On average, 1.77 PDIs (n = 3,932) were detected in each patient’s (n = 2,782) prescription. DICMA were failed to identify 363 DIs because of the unavailability of particular drugs in the drug-directory of the DICMA.
| Variable | Value | P Value |
|---|---|---|
| Number of prescriptions (patient-wise) screened by CCPs | 2,967 | |
| DIs identified in prescriptions (N = 2,967), No. (%) | 2,782 (93.76) | 0.001 |
| Number of DIs identified by DICMA | 11,128 | |
| PDIs identified in prescriptions (N = 2,782), No. (%) | 2,226 (80.01) | 0.001 |
| Number of PDIs identified and reported to physicians (N = 11,128), No. (%) | 3,932 (35.33) | |
| Average number of PDIs identified per patient | 1.77 | |
| DICMA failed to create DI suggestion (N = 2,782) | 363 |
Drug-Drug Interactions in Prescriptions
Prescriptions containing less than 6 medications and 6 to 10 medications were found with (on average) 1.38 and 3.28 DIs, respectively; whereas, in cases that the number of medications in prescription was above 10, the number of identified DIs was found to be more than the twice than that of 6 to 10 medications in each prescription (Table 2). In the case of PDIs, prescriptions containing more than 10 medications, were found with 4.7 (on average) PDIs, whereas less than 6 and 6 to 10 medications caused (on average) 0.54 and 1.42 PDIs, respectively (Table 2).
| Variable | Value (N) |
|---|---|
| Drug-drug interactions (DIs) | |
| AN of DIs per prescription (containing < 6 medications) | 1.38 |
| AN of DIs per prescription (containing 6 - 10 medications) | 3.28 |
| AN of DIs per prescription (containing > 10 medications) | 7.53 |
| Potential drug-drug interactions (PDIs) | |
| AN of PDIs per prescription (containing < 6 medications) | 0.54 |
| AN of PDIs per prescription (containing 6 - 10 medications) | 1.42 |
| AN of PDIs per prescription (containing > 10 medications) | 4.7 |
Drug-Interaction Patterns in Polypharmacy Prescriptions
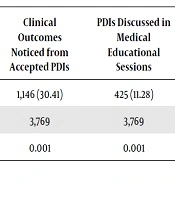
Of 3,932 DI or PDI suggested by the CCPs, physicians accepted 3,769 (95.85%) of them and took immediate corrections, accordingly (Table 3). Among the accepted suggestions, 1,146 (30.41%, n = 3,769) were found with immediate positive clinical outcomes in the patients after reconciling the prescriptions (P < 0.05) (Table 3). In the medical, educational sessions, 4,25 (11.28%; n = 3,769) selected PDIs were considered for open discussion among the multidisciplinary healthcare professionals, including physicians and critical care pharmacists (Table 3) to increase the awareness of core healthcare professionals concerning medications safety. Regarding the user-friendliness and ease of use of free DICMA, the user satisfaction level of CCPs was measured using a satisfaction scale (level was 4 on a scale of 5) (Table 4).
| PDIs Accepted by Doctors | Clinical Outcomes Noticed from Accepted PDIs | PDIs Discussed in Medical Educational Sessions | |
|---|---|---|---|
| No. (%) | 3,769 (95.85) | 1,146 (30.41) | 425 (11.28) |
| N | 3,932 | 3,769 | 3,769 |
| P value | 0.001 | 0.001 | 0.001 |
Doctors’ Acceptance of Suggestions
| Satisfaction | Scale |
|---|---|
| Very Satisfied | 5 |
| Satisfied | 4← |
| OK | 3 |
| Dissatisfied | 2 |
| Very dissatisfied | 1 |
User Satisfaction Scale
5. Discussion
When two or more drugs interact in such a manner that alters the efficacy and toxicity of one or more drugs, the resulted DI may be harmful or in some cases, beneficial for the patient, based on patients’ health status (11). In this study, CCPs identified 11,128 DIs using DICMA among 2,782 ICU-patients (on average, 1.77 PDIs were identified in each patient’s prescription). Nowadays, identified and unidentified drug-drug interactions in polypharmacy prescriptions are major therapeutic concerns for healthcare professionals and scientists worldwide (2, 12). Every year several new drugs enter the global market, that some of them may have DIs with the existing drugs (13). Sometimes patients experience mild to severe ADRs because of these DIs, which may result in unwanted events (e.g. hospitalization or prolonged hospitalization, and or increased health expenditures) (4, 13, 14). Another prospective study found that 25.9% of all reported ADRs were due to PDIs (15). The possible etiologies behind these DIs in critically ill patients are physiological changes such as altered renal and hepatic function, compromised immunity, diminished body mass, long-time bedridden, altered fluid volume status of the body, multiple medical co-morbidities, and drug-habits of prescribers (16).
Due to DIs, the pharmacological properties of the associated drugs may be altered, which may cause detrimental effects in patients. In most cases, DIs are preventable and reversible medication-related errors. The higher the number of drugs in a prescription, the greater the possibility of DIs (11, 16). In this study, the average rate of PDI was 4.7 when there were more than 10 medications in a prescription, and this figure gets down remarkably to 1.42 when 6 to 10 medications were available in each prescription. A study found that patients who take ten or more medications simultaneously have over 90% possibility to have one or more clinically significant DI (17). Another study showed that the rate of PDI among patients who took 5 medications per each prescription was 40%, and when the number of medications was 7 or more, the rate was increased to 80% (14). That is why the World Health Organization (WHO) has recommended keeping on an average 1.4 - 2.4 medications per prescription to reduce the possibility of DIs (18). Studies have reported that 2.2-30% of PDIs occur in hospitals (19) and up to 11.1% of hospitalized patients experience unwanted symptoms of PDIs (20). An Indian study demonstrated that out of 751 polypharmacy prescriptions, 706 prescriptions had PDIs (11). A prospective, observational study in South India showed that of all detected DIs, 30.67% are PDIs (21). In Brazil, a retrospective study on 299 patients showed that 68.6, 73.9, and 69.6% of patients were experienced PDIs within the first 24-hour of ICU admission, in the 50th length‐of‐stay in ICU, and at the time of discharge, respectively (1). Another cross-sectional study reported that by using compendia such as Stockley’s, Micromedex and Epocrates, 1,120 PDIs were reported among 275 patients and major PDIs were found in 18% of patients. The same study also mentioned that 79% of patients had at least one PDI in their prescriptions (22). The most common causes of PDIs include drug dose, administration method, serum drug level, drug metabolism pathways, duration of administration, and patient-related factors, such as age, gender, weight, race, and genetic predisposition (23).
CCPs, a specialized professional-brunch of clinical pharmacy, are the experts of conservative-pharmacotherapy management in critical care areas such as ICU (8). According to the American College of Physicians, CCPs are health professionals with sufficient training and enormous skills required to provide high-quality care to patients (24). A frequent DI screening program is an important process in the ICU-setups that can remarkably reduce the number of PDIs among the prescriptions of critically ill patients and during hospitalization (8, 25). A study conducted in New York reported that the presence of CCPs in the ICU’s clinical-rounds reduced the rate of DIs by 65% (24).
Fast identifying of DIs in polypharmacy prescriptions and reporting PDI information to physicians is a challenging task, even with technological supports (e.g., DICMA). Daily evaluation of patient’s prescription concerning disease status, consecutive physiological conditions, organ functions, and medical co-morbidities, as well as the screening of potential DIs by analyzing interaction-criteria of the prescription’s medications, are difficult tasks (24, 25). To date, more than 54 software, such as Micromedex® Healthcare Series, Drug Interactions Facts®, Lexi-Interact®, Pharmavista®, EpocratesRx®, MediQ® are available for CCPs or other health professional to easily check DIs in prescriptions (26). Either be performed manually or using specific DI-checker software, DIs/PDDIs-screening is a cumbersome and time-consuming process, and the risk of over-looking and wrong selection is relatively high. Nowadays, the use of smartphone-based drug-interaction software has made the DI-screening process easier for CCPs, and DI/PDI-free prescriptions can easily be assured all around the world. However, in resource-limited healthcare environments, the absence of these tools/software makes the DI-checking task very difficult for CCPs (24, 25, 27). In the current study, the hospital had no internal DI-checker software incorporated in its HIS, and the cost of purchasing or having a subscription of such an integrated software is a huge burden for the hospital authorities at that moment. Two of the most popular free DICMA (i.e., Medscape and Epocrates) are the easiest tools for mobile phone-based DI-checking. In this study, CCPs easily downloaded and installed these two DICMA on their mobile phones, and using these apps even in offline mode, they evaluated prescription of 2,782 patients (93.76%; n = 2967) and successfully reported 3,932 PDIs to physicians. With DICMA-user-satisfaction level 4 (Satisfied), CCPs identified a total of 11,128 and 3,932 DIs and PDIs, respectively, which finally resulted in medication safety for critically ill patients. When hospitals cannot afford the costs of non-free computer or mobile-based drug-interaction checker software to be used in the daily DI-screening practice of clinical pharmacists, free online or offline DICMA may be an effective alternative to sort-out DIs/PDIs within a short time among the medications of polypharmacy prescriptions and to ensure hedging the potential risks of prescribed medications for critically ill patients.
Major limitations of this study were investigating only one ICU, the clinical outcomes of patients were not considered regarding the identified drug-drug interactions, no potential drug-drug interactions versus mortality rate analysis was considered, and no prescribed medications-associated side effects analysis in the polypharmacy prescriptions of critically ill patients was performed.
5.1. Conclusions
Drug-drug interactions or potential drug-drug interactions among the prescribed medications for critically ill patients are a major health concern for all healthcare professionals, including CCPs worldwide. CCPs are professionally responsible for screening DIs/PDIs among the medications of polypharmacy prescriptions and for ensuring hazard-free medications as one of the most important patient’s rights. In resource-limited healthcare facilities, free DICMA may be an effective alternative for the rapid screening of DIs. In this study, CCPs accomplished the interaction-free safe medications by identifying DIs and PDIs in prescribed medications for critically ill patients by using free DICMA with a high level of user satisfaction.