1. Background
Scorpion envenomation is a major medical issue in many countries, which threatens a population of about 2.3 billion (1). Iran has the highest number of scorpion stings in the world after Mexico (2-4). Of the 52 species of scorpions identified in the Middle East, the most dangerous ones have been found in Iran (4). Scorpion stings have been reported in all provinces of Iran, however, its highest incidence has been in Khuzestan so that its occurrence reaches 541 cases per 100,000 people (5, 6). According to the most recent classification of scorpions in Iran, three families of Buthidae, Scorpionidae, and Hemiscorpiidae have been reported from all over the country (3) Hemiscorpius lepturus, which belongs to the Arachnida class, Scorpiones order, Scorpionoidea superfamily, and Hemiscorpiidae family, is the most critical scorpion in Khuzestan, Iran and the world (7). The Hemiscorpiidae family is found in six Middle Eastern countries, including Iran, Iraq, Pakistan, Saudi Arabia, Oman, Yemen, and United Arab Emirates (8). Although only 15% of scorpion stings were due to this species, it was the source of 89% of mortalities following scorpion envenomation (9). Hemiscurpius are non-digger scorpions which have sexual dimorphism so that the body length of the male is longer than that of the female (8 and 5 cm, respectively). They have a transparent to turbid yellow color with brown spots at the tip of the legs. The moving finger of chelicera has two branches (10). The venom of H. lepturus has a range of toxic properties, including cytotoxicity, nephrotoxicity, hepatotoxicity, and hemolysis, which can lead to acute renal failure, leukopenia, thrombocytopenia and microangiopathic hemolytic anemia, as complications following scorpion sting (4, 9, 11, 12).
The venom of many arthropods, including scorpions, was proved to induce hemostatic disorders (13). In contrast, very few studies have been performed on the coagulopathic properties of scorpion venoms and the published data on the subject is inadequate.
Coagulation disorders, including bleeding diathesis and disseminated intravascular coagulation (DIC) expressed as prolongation of prothrombin time (PT) and activated partial thromboplastin time (APTT) were also reported as a complication after being stung by H. lepturus in severe forms (9, 14). However, the venom components responsible for coagulopathies and their action mechanisms are so far unknown.
2. Objectives
Since some of the scorpion venom fractions exclusively affect specific systems or organs in the body, one of the primary goals of this study was to recognize H. lepturus venom fractions whose target is the coagulation system. In addition to the study of fractions, a better understanding of possible mechanisms and the move towards the provision of specific anti-venom as an alternative to polyvalent anti-venom.
3. Methods
3.1. Venom Preparation
H. lepturus scorpions were harvested over nocturnal UV light inspection from different parts of Khuzestan province in the South-west of Iran. The scorpions were milked by the electrical stimulation of telson after the precise identification of species by morphological key diagnosis (15). The venom was water purified, centrifuged, freeze-dried, and stored at -20 °C until used (16). The freeze-dried venom (100 mg) was dissolved in 10 ml ammonium acetate Buffer (pH: 8.6) and then dialyzed against distilled water at 4 °C for 48 hours. After dialysis, the venom solution was centrifuged to separate soluble proteins from the insoluble mucoproteins (Sigma 6K15®, UK) at 14,000 rpm for 17 minutes, and the supernatant was collected.
3.2. Venom Fractionation
Venom fractionation was performed by Gel filtration chromatography. In brief, venom prepared was eluted with 0.1 M ammonium acetate buffer (pH 8.6) on Sephadex G-50 column (2.5×125 cm). After loading the venom on the column, elution buffer was gathered in an outflow of 45 ml/hour. Fractions were collected in 4 ml volumes and were identified by UV spectrophotometer (280 nm), pooled, and lyophilized.
3.3. Protein Determination
Protein concentration of whole venom and each fraction were measured by the Bradford method, and Bovine Serum Albumin (BSA) was utilized as a standard.
3.4. SDS–PAGE
Assessment of derived purified fractions from Gel filtration chromatography as well as whole venom was performed by SDS-PAGE with 5% and 12% stacking and resolving gels, respectively, to obtain protein profiles according to Laemmli, 1970 (17).
3.5. Animals
A total of 96 Albino male Wistar rats with 250-300 g weight were kept in a room with a temperature upheld at 24 ± 2 ºC and a humidity of 55 ± 5%, with a 12-h light/12-h dark illumination cycle. The rats were fed a commercial laboratory pellet diet and tap water ad libitum. All experiments were carried out according to ethical rules for care and use of laboratory animals, and were approved by the Experimental Animals Committee of Shahid Chamran University of Ahvaz, Iran (Ethics code: EE/98.3.02.88214/scu.ac.ir).
3.6. Experimental Design
This prospective randomized experimental study was conducted from June to August 2019. Given that six venom fractions were extracted from whole venom through chromatography, the study was performed on eight equal groups (12 rats in each group) and were treated as follows:
Control group: 0.5 ml normal saline intraperitoneally (IP).
Whole venom group: H. lepturus whole venom at a dose of 1 mg/kg body weight IP.
F1 to F6 groups: Isolated fractions 1 to 6 of H. lepturus venom at a dose of 0.12, 0.43, 0.08, 0.18, 0.06, and 0.13 mg/kg body weight IP, respectively.
The selected dose for each of the fraction-receiving groups was proportional to the concentration of the isolated fraction relative to the whole venom.
3.7. Blood Collection
Sampling was performed at 1, 3, and 24 hours after venom/saline injection; four rats from each group were sampled every time. Blood samples were collected into sodium citrate containing tubes via cardiac puncture. Immediately after sampling, plasma samples, harvested by centrifugation, were subjected to coagulation/fibrinolysis assessments. It should be noted that hemolytic plasma samples were excluded from the study and repeated.
3.8. Fibrinogen Assessment
Plasma fibrinogen concentration was determined by Mahsa-Yaran kit, Iran, following the manufacturer's instructions.
3.9. Prothrombin time (PT) and Activated Partial Thromboplastin Time (APTT)
In this study, PT and APTT were determined in citrated plasma samples using PT and APTT reagents (Thermo Fisher Scientific, USA) following the manufacturer's instructions, respectively.
3.10. Fibrinogen Degradation Product (FDP) and D-dimer
Plasma FDPs and d‐dimers were measured via competitive ELISA technique using rat specific commercial kits (Kamiya Biomedical Co., Cat. No. KT-59925 and KT-13053, Seattle, WA) according to the manufacturer's instructions.
3.11. Statistical Analysis
The data were analyzed using Analysis of variance (ANOVA) and Tukey post-hoc tests by SPSS software version 16 (Chicago, USA). The values were stated as mean ± standard deviation, and P value < 0.05 was considered statistically significant.
4. Results
4.1. Venom Fractions
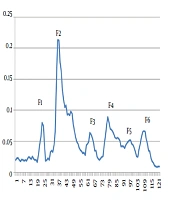
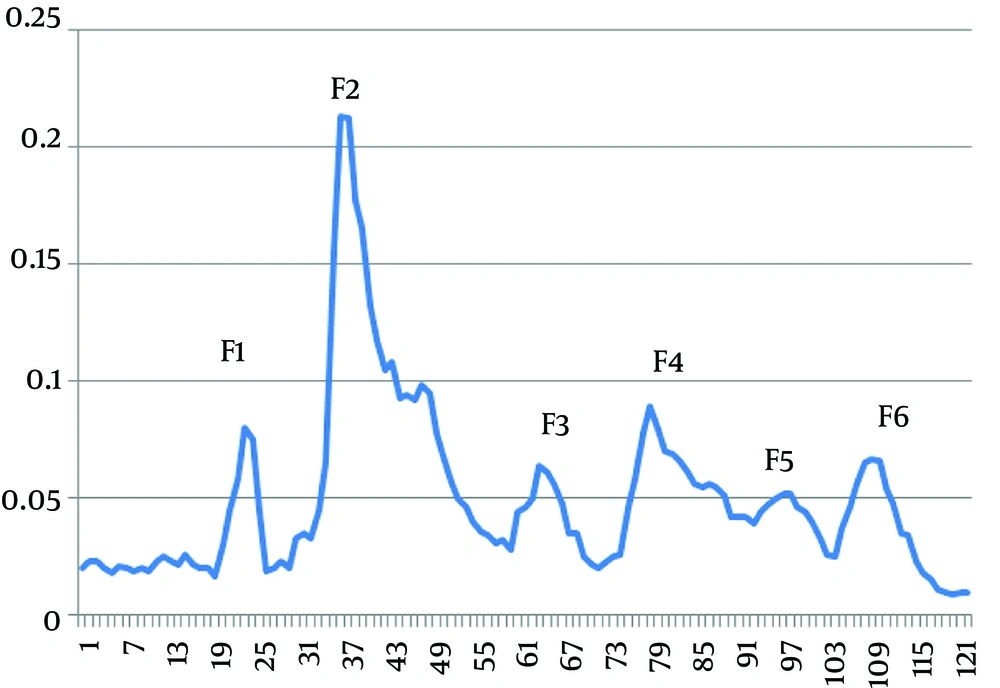
The injection of 10 ml soluble whole venom of H. lepturus scorpion onto Sephadex G-50 provided six fractions as F1, F2, F3, F4, F5, and F6 at optical density of 280 nm (Figure 1). The protein concentration and rate of yield of each fraction are summarized in Table 1.
| Venom Fractions | ||||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | |
| Protein (mg) | 8.04 | 28.81 | 5.36 | 12.06 | 4.02 | 8.71 |
| Yields (%) | 12 | 43 | 8 | 18 | 6 | 13 |
Protein Content and Yield of Fractions of H. lepturus Venom
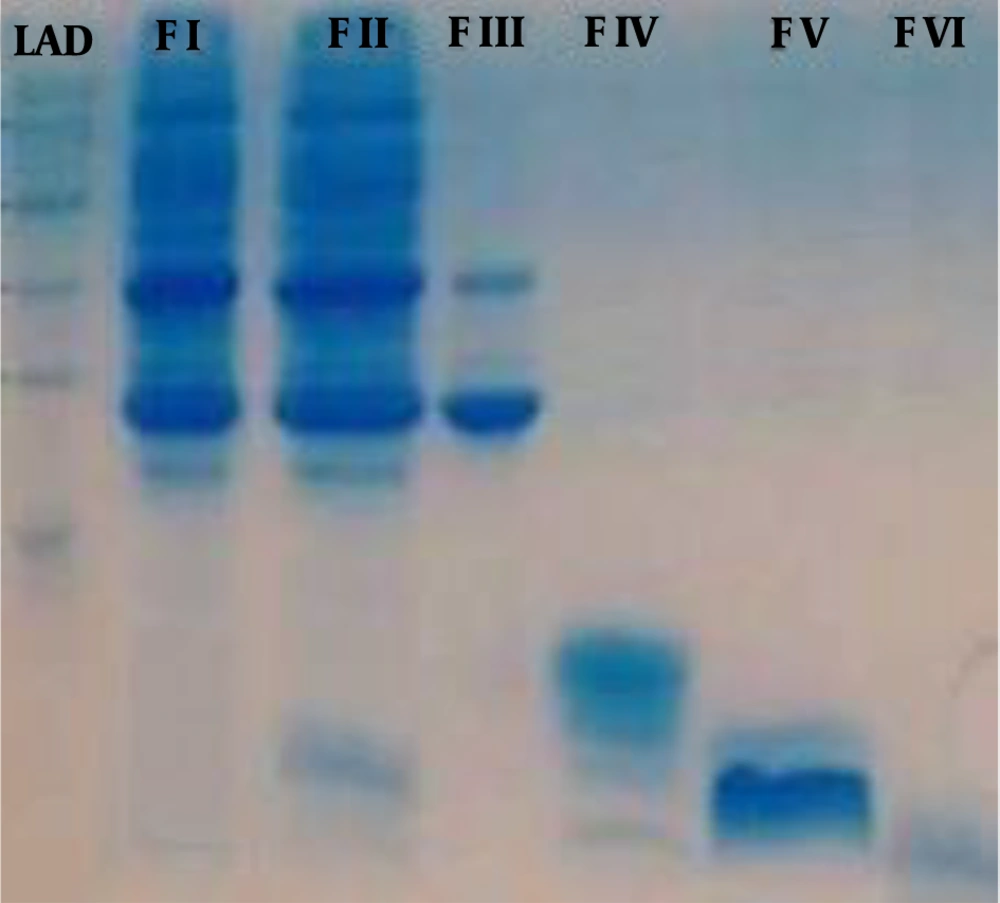
According to SDS-PAGE, the venom proteins were estimated between ≤ 5 and ≤ 160 kDa. The molecular weight of F1 was in the range of 25 to 160 kDa. The proteins isolated in the fraction F2 had a molecular weight of about 8 to 25 kDa with four detectable bands of 8, 14, 22, and 25 kDa. The molecular weight of F3 was between 8 to 14 kDa. Bands less than 5 kDa were observed in F4, F5, and F6 fractions (Figure 2).
Analysis of H. lepturus Scorpion of Venom Fractions by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis. LAD: 250-1 kDa Ladder, F I: venom fraction I (MW: 160 to 25 kDa), F II: venom fraction II has 4 bands (MW: 25-22-14 and 8 kDa), F III: venom fraction III has 2 bands (MW: 14 and 8 kDa), F IV: venom fraction IV, V, VI has bands less 5 kDa.
4.2. Fibrinogen Concentration
Plasma fibrinogen concentration was significantly elevated in whole venom group (P value < 0.05), while it was significantly decreased in F3, F5, and F6 groups at the first sampling time and in F3 and F5 group at the second sampling, in comparison to the control group (P value < 0.05) (Table 2).
| Group | Sampling Times | ||
|---|---|---|---|
| 1H | 3H | 24H | |
| Control | 279.67 ± 5.33A | 225.00 ± 15.00B | 226.00 ± 15.87AB |
| Crud Venom | 347.00 ± 14.15A* | 272.67 ± 21.95B | 310.33 ± 12.66AB |
| F1 | 340.33 ± 20.11 | 275.67 ± 14.25 | 283.33 ± 23.39 |
| F2 | 276.67 ± 18.09 | 251.33 ± 11.40 | 295.33 ± 26.77 |
| F3 | 221.33 ± 11.56A* | 171.00 ± 2.00B* | 190.67 ± 14.76AB |
| F4 | 269.67 ± 8.66A | 172.00 ± 16.07B | 175.00 ± 27.00AB |
| F5 | 146.33 ± 10.08AB* | 135.67 ± 4.48A* | 173.33 ± 7.21B |
| F6 | 177.33 ± 5.54* | 183.00 ± 11.93 | 237.00 ± 32.00 |
4.3. PT Analysis
PT assessment revealed that there was only a significant increase in the group F6 compared to the control group at 1 and 3 hours after envenomation (P value < 0.05). At 24 hours, whole venom and fraction 2, 5, and 6 receiving-groups showed an increased PT value, while fraction 3 and 4 resulted in a shortened PT in comparison to the control group (P value < 0.05) (Table 3).
| Group | Sampling Times | ||
|---|---|---|---|
| 1H | 3H | 24H | |
| Control | 11.65 ± 0.11 | 12.74 ± 0.22 | 13.32 ± 0.25 |
| Crud Venom | 13.40 ± 0.31A | 13.59 ± 1.11A | 17.41 ± 1.00B* |
| F1 | 14.70 ± 0.34 | 14.78 ± 0.40 | 15.40 ± 0.69 |
| F2 | 15.57 ± 0.38 | 15.86 ± 0.30 | 16.24 ± 0.38* |
| F3 | 9.05 ± 0.41 | 8.68 ± 0.29 | 8.49 ± 0.22* |
| F4 | 8.43 ± 0.10AB | 8.06 ± 0.15A | 8.76 ± 0.18B* |
| F5 | 19.00 ± 0.54A | 15.33 ± 0.88B | 17.94 ± 0.82AB* |
| F6 | 47.68 ± 7.53* | 34.33 ± 12.86* | 17.92 ± 1.07* |
4.4. APTT Analysis
APTT was significantly increased in group whole venom, F5, and F6, while it was decreased in group F3 and F4 compared to the control group at one hour after venom injection (P value < 0.05). In addition, there was a significant increase in the group F6 at three hours and in groups whole venom, F5 and F6 at 24 hours after envenomation, while APTT was decreased in the group F4 at the same time in comparison to the control group (P value < 0.05) (Table 4).
| Group | Sampling Times | ||
|---|---|---|---|
| 1H | 3H | 24H | |
| Control | 30.66 ± 0.57 | 31.52 ± 0.70 | 31.80 ± 0.73 |
| Crud Venom | 41.19 ± 1.96* | 42.55 ± 0.48 | 42.77 ± 0.13* |
| F1 | 30.78 ± 0.36 | 29.33 ± 1.78 | 28.65 ± 1.06 |
| F2 | 27.61 ± 0.70 | 28.28 ± 1.16 | 27.77 ± 0.52 |
| F3 | 20.33 ± 1.33A* | 35.70 ± 2.60B | 22.86 ± 1.61A |
| F4 | 19.47 ± 0.28* | 29.21 ± 5.46 | 22.00 ± 4.04* |
| F5 | 58.66 ± 1.33A* | 27.66 ± 6.66B | 49.76 ± 4.27A* |
| F6 | 66.00 ± 6.02A* | 60.17 ± 0.17AB* | 49.66 ± 1.45B* |
4.5. FDP Analysis
The concentration of FDP was significantly increased in whole venom and F2 group compared to the control group at all sampling times (P value < 0.05) (Table 5).
| Group | Sampling Times | ||
|---|---|---|---|
| 1H | 3H | 24H | |
| Control | 6.45 ± 0.11 | 6.08± 0.16 | 5.75 ± 0.45 |
| Crud Venom | 26.16 ± 0.53A* | 35.42 ± 0.44B* | 14.97 ± 1.15C* |
| F1 | 7.98 ± 0.42A | 6.61 ± 0.58AB | 5.64 ± 0.15B |
| F2 | 16.57 ± 0.45* | 19.28 ± 0.39* | 15.14 ± 1.77* |
| F3 | 5.48 ± 0.21 | 6.54 ± 0.02 | 6.07 ± 0.36 |
| F4 | 5.86 ± 0.69 | 5.63 ± 0.14 | 6.14 ± 0.10 |
| F5 | 7.36 ± 0.66 | 7.47 ± 0.04 | 6.05 ± 0.25 |
| F6 | 6.45 ± 0.29 | 6.29 ± 0.32 | 6.18 ± 0.26 |
4.6. D-Dimer Analysis
The injection of H. lepturus whole venom and venom fraction F2 resulted in a significant elevation in plasma D-dimer concentration compared to the control group at all sampling times (P value < 0.05) (Table 6).
| Group | Sampling time | ||
|---|---|---|---|
| 1H | 3H | 24H | |
| Control | 0.50 ± 0.09 | 0.43 ± 0.01 | 0.54 ± 0.05 |
| Crud Venom | 3.97 ± 0.13A* | 8.22 ± 0.45B* | 2.13 ± 0.46C* |
| F1 | 0.43 ± 0.02 | 0.51 ± 0.01 | 0.46 ± 0.06 |
| F2 | 3.02 ± 0.16A* | 7.01 ± 0.48B* | 1.81 ± 0.32A* |
| F3 | 0.58 ± 0.01A | 0.79 ± 0.11B | 0.69 ± 0.03A |
| F4 | 0.74 ± 0.06 | 0.77 ± 0.09 | 0.76 ± 0.09 |
| F5 | 0.77 ± 0.14 | 0.74 ± 0.06 | 0.63 ± 0.00 |
| F6 | 0.76 ± 0.12 | 0.73 ± 0.03 | 0.60 ± 0.03 |
5. Discussion
This experimental study was performed to investigate the effects of fractions of H. lepturus venom on the hemostatic system in rats.
In the present study, plasma fibrinogen analysis revealed an elevation following whole venom, F1, and F2 injection and a decline in the rest of the groups (F3, F4, F5, and F6). Hyperfibrinogenemia, observed in this experiment, might be attributed to the thrombin-like enzyme activity of whole venom and its higher molecular weight components (F1 and F2). In support of this theory, several enzymatic proteins and peptides, including metalloproteinases, phospholipases A2 (PLA2s), and serine proteases, were identified in scorpion venoms, comprising H. lepturus, that can affect the coagulation system (18, 19).
In contrast, fibrinogen decline caused by lower molecular weight fractions of H. lepturus venom (F3 to F6) in this study, is probably due to their antithrombin properties or thrombotic conditions.
Anticoagulant peptides and phospholipases with thrombin inhibiting activity were previously isolated from various scorpion venoms (20). Additionally, low molecular weight peptides with phospholipase activity and anticoagulant effects have already been isolated from H. lepturus venom (21). These results suggest that the inhibitory effects of above-mentioned fractions on thrombin/ fibrinogen may be due to their phospholipase properties (F5 and F6) or a mechanism independent of their enzymatic activity (F3 and F4).
In the present experiment, PT results were prolonged in all envenomated groups except for F3 and F4 groups. Additionally, in APTT analysis, a significant extension was noted in whole venom, F5, and F6 group while it was significantly shortened in the groups F3 and F4.
F1 and especially F2 fragment, in this study, prolonged PT which indicates that some clotting factors within the extrinsic pathway are deficient or inhibited. However, whole venom, F5, and F6 resulted in the extension of both PT and APTT, implying the disturbances in both the intrinsic and the extrinsic pathways or in the final common pathway. Hence, the thrombin-inhibiting properties of these two fragments might be, at least in part, responsible for the changes in coagulation tests.
Additionally, various venoms contain phospholipase A2 (PLA2s) enzymes in a range of forms with a discrepancy in their capacity to alter hemostasis (22).
On the other hand, a potent phospholipase protein, called Hemilipin, has already been separated from H. lepturus scorpion venom (21). Moreover, H. lepturus venom phospholipase activity was found to be concentration-dependent (23). Considering the molecular weight of Hemilipin that is in the range of fraction 2 of this study (25-8 kDa) and also due to the higher concentration of this fraction than other venom components, some of the anticoagulant effects of H. lepturus venom, detected in the present experiment, are likely due to phospholipases. In addition, some peptides with less than 10 kDa weight were formerly isolated from H. lepturus venom, which were categorized as PLAs (24). Thus, for F5 and F6 fractions with low molecular weight in this survey, a mechanism similar to the preceding ones can be considered.
Conversely, F3, and F4 fragments resulted in shortening PT and APTT in the present study. These effects might be related to proteolytic characteristics of venom components, which act as serine protease (8). Similar effects were reported for Palamneus gravimanus scorpion venom, having both procoagulant and anti-coagulant properties (25). In the present study, the concentration of FDP and D-dimer followed the same pattern in that there was a significant elevation in whole venom and F2-receiving groups.
A severely elevated D-dimer and FDP levels in this experiment reflect widespread activation of the coagulation and subsequent fibrinolysis and have a high predictive value for venous thromboembolism (VTE), which may occur either locally or diffusely (26).
Thromboembolic disorders were reported as a complication in severe forms of H. lepturus envenomation in human victims (9, 14, 27). Fibrin(ogen)olytic components of venoms were considered the most probable cause of the mentioned hemostatic syndromes.
Fibrin(ogen)olytic enzymes have been categorized into two classes, serine proteases and metalloproteases (28) both of which were previously identified in H. lepturus venom gland (18, 24, 29). Similar enzymes with fibrin(ogen)olytic and plasminogen activator-like potential were also purified from the venom of Tityus discrepans (28) and Androctonus crassicauda (30).
The highest fibrinolytic activity in the present study belonged to whole venom followed by fraction 2. In this investigation, F2 injection resulted in PT prolongation and fibrinogen decline, as well. Furthermore, it is likely that the possible fibrinogen degradation by H. lepturus venom was associated with loss of fibrinogen coagulant activity. This may also partially explain the prolongation of coagulation tests caused by the whole and F2 fraction of the scorpion venom noticed in this experiment. Coagulopathy with PT prolongation and increased D-dimer was formerly reported in scorpion sting victims (31). Similar findings were also documented with T. discrepans and A. crassicauda scorpion venoms (28, 30).
Although the molecular mass of proteolytic compounds in the above-mentioned scorpion venoms was higher than the F2 fraction of the present study, there is still some evidence of the presence of enzymes of the same molecular weight range with metalloprotease properties in H. lepturus venom (18). Therefore, according to the present findings and previous studies, it is likely that the fibrin (ogen) olytic effects of whole venom and fraction 2 are due to the metalloproteases present in the scorpion venom.
It should be noted that further separation of toxin fractions by HPLC chromatography could more accurately identify the fractions affecting the coagulation system. However, this method was not performed in the present study.
In conclusion, in total, H. lepturus venom appears to have both procoagulant and anticoagulant properties, which are distinct characteristics of it. Procoagulant and fibrin (ogen) olytic activities of whole venom and the higher molecular weight fractions are likely due to proteolytic enzymatic components, while anticoagulant effects of smaller peptides might be attributed to phospholipases or nonenzymatic inhibitory effects. Therefore, serious coagulation disorders caused by the venom of this scorpion should be considered by the medical staff, especially in emergency cases. Moreover, identifying the different hemostatic effects of various fractions of the venom can be helpful in preparing a more specific anti-venom to prevent or treat the coagulopathies following this scorpion sting.