1. Background
Urinary Tract Infections (UTIs) are one of the most common acquired bacterial infections. It has been estimated that approximately 150 million people worldwide develop UTIs annually, with high costs, including medical and hospitalization expenses (1). Recent studies conducted in Iraq found that E. coli was the most common uropathogen among Gram-negative isolates and Staphylococcus species among Gram-positive bacteria causing UTIs (2, 3).
The prevalence rate of uropathogens varies depending on the age and gender of patients, hospitalization, catheterization, and previous uses of antibiotics (4-6). Besides, UTIs occur more commonly in the female gender than in the male gender mainly due to the shorter urethra that allows pathogens to travel more easily into the bladder and sexual activity that increases the risk of uropathogens (7). It is worthwhile to mention that persistent symptoms for at least one week could increase the risk of recurrent infections because of the occult kidney infection, particularly among females with low socioeconomic status (7).
The emergence of antibiotic resistance is a serious public health concern. Overuse of antimicrobial agents in the community without medical prescription and their low costs are thought to have contributed to the emergence and proliferation of antimicrobial resistance (8). It was previously observed that the increasing resistance rate to commonly used antimicrobial drugs could also play a major role in the occurrence of complicated and recurrent infections (9). Furthermore, differences in antibiotic resistance rates were documented in different geographical and regional locations (2, 10). Therefore, the periodic evaluation of the frequency of pathogens with their antibiotic resistance patterns is recommended in different countries for therapeutic advice and antimicrobial resistance prevention (11). Additionally, investigating antimicrobial susceptibility patterns could help allocate UTI empirical treatment guidelines in different regions.
2. Objectives
The current study aimed to investigate the most common bacterial uropathogens and their antibiotic sensitivity patterns among females suffering from UTIs in Duhok province, Iraq.
3. Methods
3.1. Sample Collection and Processing
A cross-sectional study was conducted in Duhok province, Kurdistan Region, Iraq, from January 2017 to February 2020. A total of 530 urine samples were collected from females referring to private clinical health centers. The age of the patients ranged from 10 to 65-years-old. Clean catch midstream urine (4 - 5 ml) was obtained in sterile disposable containers from patients to avoid contamination, and the containers were transferred immediately to the Duhok private clinical laboratory.
A loopful of urine sample was cultured on MacConkey and Blood agar (Oxoid Ltd., Bashingstore, Hampshire, UK), incubated overnight at 37°C and inspected for the presence of bacterial growth. Plates without any colony at the end of the 24 h incubation period were further extended for 48 h. Agar plates with a colony count of equal to or more than 105 CFU/ml of urine indicated significant bacteriuria and evidence of UTIs. The inclusion criteria included female gender, positive microbiological evidence of UTIs, and agreement to be recruited in the study.
3.2. Bacterial Identification and Antimicrobial Sensitivity
Purified colonies were initially classified using Gram staining and then identified based on standard microbiological cultures and biochemical characteristic reactions according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (12, 13). The Vitek-2 system (bioMerieux, US) was used for antimicrobial susceptibility testing. The antimicrobial susceptibility pattern of the isolates was performed by using the Vitek-2 system (bioMerieux, US).
3.3. Ethics Statement
The study and the protocol of attaining consent were approved by the scientific committee at the College of Medicine, University of Zakho, Kurdistan, Iraq. Formal consent was obtained from the patients recruited in this study. Only participants who gave their consent before sample collection were included in the study.
4. Results
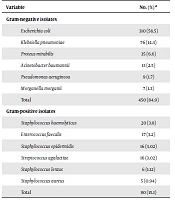
In this study, 530 female subjects clinically infected with UTIs who met the inclusion criteria were recruited in the study. Of them, 450 (84.9%) were positive for Gram-negative bacteria and 80 (15.1%) for Gram-positive bacteria (Table 1). The results showed that E. coli was the most common Gram-negative uropathogen (n = 310, 58.5%), followed by K. pneumoniae (14.3%), while M. morganii recorded the lowest rate (1.3%) (Table 1). On the other hand, the most common Gram-positive uropathogen was S. haemolyticus (3.8%), followed by E. faecalis (3.2%), while S. aureus showed the lowest rate (0.94%) (Table 1).
| Variable | No. (%) a |
|---|---|
| Gram-negative isolates | |
| Escherichia coli | 310 (58.5) |
| Klebsiella pneumoniae | 76 (14.3) |
| Proteus mirabilis | 35 (6.6) |
| Acinetobacter baumannii | 13 (2.5) |
| Pseudomonas aeruginosa | 9 (1.7) |
| Morganella morganii | 7 (1.3) |
| Total | 450 (84.9) |
| Gram-positive isolates | |
| Staphylococcus haemolyticus | 20 (3.8) |
| Enterococcus faecalis | 17 (3.2) |
| Staphylococcus epidermidis | 16 (3.02) |
| Streptococcus agalactiae | 16 (3.02) |
| Staphylococcus lentus | 6 (1.12) |
| Staphylococcus aureus | 5 (0.94) |
| Total | 80 (15.1) |
Distribution of Bacterial Profiles in Urine Samples in this Study (n = 530)
Regarding antibiotic susceptibility patterns, E. coli isolates were the predominant uropathogens and showed high resistance to ampicillin (84.8%), aztreonam (58.7%), ceftriaxone (58.1%), and cefepime (57.4%) and high sensitivity to ertapenem (98.7%), imipenem (97.7%), and amikacin (92.9%) (Table 2). Besides, K. pneumonia and P. mirabilis displayed a similar resistance pattern as for E. coli and showed high susceptibility to ertapenem and imipenem and high resistance to ampicillin, aztreonam, ceftriaxone, and cefepime (Table 2). In terms of the sensitivity pattern of A. baumannii isolates, it was revealed that 92% of the isolates were resistant to ampicillin, amoxicillin/clavulanic acid, cefazolin, and cefoxitin and highly sensitive to trimethoprim/sulfamethoxazole (92.3%) (Table 2). About 78% of the P. aeruginosa isolated were resistant to ampicillin and nitrofurantoin, while they were highly susceptible to ertapenem (77.8%) (Table 2). Additionally, M. morganii isolates were absolutely sensitive to cefepime, aztreonam, ertapenem, imipenem, amikacin, ciprofloxacin, levofloxacin, and nitrofurantoin (100%) while expressed very high resistance to ampicillin (100%) (Table 2).
| Antibiotics | Number of Isolates (Resistance Percentages) | |||||
|---|---|---|---|---|---|---|
| E. coli | K. pneumoniae | P. mirabilis | A. baumannii | P. aeruginosa | M. morganii | |
| Ampicillin | 263 (84.8) | 72 (94.7) | 29 (80.6) | 12 (92.3) | 7 (77.8) | 7 (100) |
| Amoxicillin/Clavulanic acid | 126 (40.6) | 30 (39.5) | 10 (27.8) | 12 (92.3) | 6 (66.7) | 6 (85.7) |
| Piperacillin/Tazobactam | 118 (38.1) | 16 (21.1) | 8 (22.2) | 7 (53.8) | 3 (33.3) | 3 (42.8) |
| Cefazolin | 113 (36.5) | 38 (50) | 18 (50) | 12 (92.3) | 6 (66.7) | 4 (57.1) |
| Cefoxitin | 142 (45.8) | 28 (36.8) | 13 (36.1) | 12 (92.3) | 5 55.6) | 3 42.9) |
| Ceftriaxone | 180 (58.1) | 48 (63.2) | 26 72.2) | 8 (61.5) | 6 (66.7) | 1 (14.3) |
| Cefeprime | 178 (57.4) | 46 (60.5) | 26 (72.2) | 9 (69.2) | 4 44.4) | 0 (0) |
| Aztreonam | 182 (58.7) | 55 (72.4) | 32 (88.9) | 10 (76.9) | 6 (66.7) | 0 (0) |
| Ertapenem | 4 (1.3) | 2 (2.6) | 1 (2.8) | 4 (30.8) | 2 (22.2) | 0 (0) |
| Imipenem | 7 (2.3) | 3 (3.9) | 1 (2.8) | 6 (46.2) | 3 (33.3) | 0 (0) |
| Amikacin | 22 (7.1) | 20 (26.3) | 6 (16.7) | 4 (30.8) | 2 (22.2) | 0 (0) |
| Gentamicin | 68 (21.9) | 12 (15.8) | 7 (19.4) | 7 (53.8) | 3 (33.3) | 1 (14.3) |
| Tobramycin | 85 (27.4) | 14 (18.4) | 9 (25) | 6 (46.2) | 3 (33.3) | 1 (14.3) |
| Ciprofloxacin | 120 38.7) | 15 (19.7) | 10 (27.8) | 10 (76.9) | 6 (66.7) | 0 (0) |
| Levofloxacin | 126 (40.6) | 14 (18.4) | 9 (25) | 8 (61.5) | 5 (55.6) | 0 (0) |
| Nitrofurantoin | 56 (18.1) | 20 (26.3) | 22 (61.1) | 11 (84.6) | 7 (77.8) | 0 (0) |
| Trimethoprim/ Sulfamethoxazole | 163 (52.6) | 37 (48.7) | 21 (58.3) | 1 (7.7) | 4 (44.4) | 6 (85.7) |
Antimicrobial Susceptibility Patterns of Gram-Negative Uropathogens
In terms of Gram-positive bacteria, Staphylococcus spp. were responsible for about 8.9% of UTI cases. Among them, S. haemolyticus as the most frequent uropathogen showed high resistance to benzylpenicillin (100%), oxacillin (95%), and erythromycin (95%) and high sensitivity to nitrofurantoin and tigecycline (100%) (Table 3). Similarly, S. epidermidis was highly resistant to erythromycin (100%), benzylpenicillin (87.5%), and oxacillin (81.3%), but highly sensitive to moxifloxacin, linezolid, and nitrofurantoin (100%) (Table 3). On the other hand, E. faecalis susceptibility to tigecycline and nitrofurantoin was high (100%), but it was highly resistant to erythromycin (100%) and clindamycin (100%). The resistance rates of S. agalactiae, S. lentus, and S. aureus to all the 16 selected antimicrobial agents are summarized in Table 3.
| Antibiotics | Number of Isolates (Resistance Percentages) | |||||
|---|---|---|---|---|---|---|
| S. haemolyticus | S. epidermidis | E. faecalis | S. agalactiae | S. aureus | S. lentus | |
| benzylpenicillin | 20 (100) | 14 (87.5) | 13 (76.5) | 7 (43.8) | 5 (100) | 6 (100) |
| Oxacillin | 19 (95) | 13 (81.3) | 15 (88.2) | 8 (50) | 3 (60) | 5 (83.3) |
| Gentamicin | 4 (20) | 2 (12.5) | 6 (35.3) | 6 (37.5) | 0 (0) | 2 (33.3) |
| Tobramycin | 4 (20) | 6 (37.5) | 7 (41.2) | 9 (56.3) | 1 (20) | 2 (33.3) |
| levofloxacin | 5 (25) | 5 (31.3) | 7 (41.2) | 8 (50) | 0 (0) | 2 (33.3) |
| Moxifloxacin | 1 (5) | 0 (0) | 9 (52.9) | 7 (43.8) | 0 (0) | 1 (16.7) |
| Erythromycin | 19 (95) | 16 (100) | 17 (100) | 16 (100) | 3 (60) | 5 (83.3) |
| Clindamycin | 8 (40) | 6 (37.5) | 17 (100) | 12 (75) | 4 (80) | 3 (50) |
| Linezolid | 1 (5) | 0 (0) | 2 (11.2) | 0 (0) | 1 (20) | 1 (16.7) |
| Teicoplanin | 3 (15) | 3 (18.8) | 3 (17.6) | 5 (31.3) | 1 (20) | 2 (33.3) |
| Vancomycin | 5 (25) | 3 (18.8) | 1 (5.9) | 2 (12.5 | 1 (20) | 2 (33.3) |
| Tetracycline | 12 (60) | 7 (43.8) | 17 (100) | 13 (81.3) | 1 (20) | 2 (33.3) |
| Tigecycline | 0 (0) | 1 (6.3) | 0 (0) | 1 (6.3) | 1 (20) | 1 (16.7) |
| Nitrofurantoin | 0 (0) | 0 (0) | 0 (0) | 5 (31.3) | 0 (0) | 1 (16.7) |
| Rifampicin | 2 (10) | 3 (18.8) | 9 (52.9) | 10 (62.5) | 1 (20) | 3 (50) |
| Trimethoprim/Sulfamethoxazole | 7 (35) | 3 (18.8) | 11 (64.7) | 4 (25) | 2 (40) | 1 (16.7) |
Antimicrobial Susceptibility Patterns of Gram-Positive Uropathogens
5. Discussion
Urinary tract infection is considered to be one of the most common bacterial infections that affect people in the community and hospitals worldwide (1). In general, females are more likely to get a UTI than males because females have a shorter urethra, so pathogens have a shorter distance to travel to reach the bladder (7). Approximately one in every three females requires antimicrobial treatment for a UTI by the age of 24, and 40 - 50% of females may suffer from UTIs during their lifetime (14).
As UTI is a very common disease in females, its diagnosis and treatment have important implications for patient’s health, healthcare costs, and antibiotic resistance development (15). Prevalence studies on local UTI pathogens and their susceptibility patterns to antimicrobial agents are considered useful to guide empirical antibiotic therapy because the prevalence of uropathogens and their features could vary with time and geographical regions (11). Therefore, the present study investigated the distribution and antimicrobial susceptibility of bacterial uropathogens in patients with UTIs. In agreement with other studies, we found that Gram-negative bacteria were common than others (3, 12, 16).
It is well known that the spectrum of pathogens isolated from patients suffering from a UTI is nearly stable, and E. coli remains the most common prevalent etiological agent (2, 17). The current study showed that E. coli was the predominant uropathogen in females in our locality. This was in agreement with the results obtained by other investigators in Iraq (2, 3). In the present study, E. coli accounted for 58.5% of the Gram-negative isolates, which is well comparable with the rates reported from outpatient studies conducted in Iran as 65.2% (18) and 51.5% (17). In another study conducted in Turkey, recruiting 429 females aged 18 to 65-years-old, E. coli (71.3%) was found to be the most common etiological agent of UTIs (16). In addition, K. pneumoniae was the second most common Gram-negative uropathogen recorded as 14.3%. In agreement with other studies, a study reported that Klebsiella spp. were observed as the second most common agents causing UTIs (26%) (18). Other frequent Gram-negative isolates included P. mirabilis, P. aeruginosa, M. morganii, and A. baumannii. This was in agreement with several previous studies that reported that these pathogens caused less frequent infections in females suffering from UTIs (2, 3, 19). The major contributing factor to the higher infection rates of E. coli in females could be due to poor genital hygienic practices by them. Because the most infecting sources of uropathogens are commensals of perianal and vaginal regions, an emphasis on personal hygiene, particularly in females, could be essential to reduce the prevalence rate of uropathogens.
Concerning antimicrobial susceptibility testing, it must be noted that significant changes in bacterial susceptibility patterns have been established over the last two decades (20). In several countries, for example, the average resistance rates of uropathogen isolates, especially E. coli, for ampicillin have increased by more than 50% (20). We found that E. coli was highly resistant to ampicillin (84.8%) and moderately resistant to aztreonam (58.7%), ceftriaxone (58.1%), and cefepime (57.4%). These results were similar to previous studies conducted in the USA (21), Iran (12), Turkey (16), and Iraq (19). On the other hand, most E. coli uropathogens were sensitive to ertapenem (98.7%), imipenem (97.7%), and amikacin (92.9%) in the current study.
Furthermore, K. pneumoniae demonstrated similar resistance patterns to E. coli and showed high sensitivity to ertapenem and imipenem and high resistance to ampicillin, aztreonam, ceftriaxone, and cefepime. Additionally, the sensitivity of P. mirabilis isolates to ampicillin, ceftriaxone, cefeprime, aztreonam, and nitrofurantoin was between 60 and 89% in the current study, which was similar to those reported in a study in Taiwan (22). In this study, the antibiotic sensitivity patterns of A. baumannii, P. aeruginosa, and M. morganii were comparable with previous studies conducted in Ethiopia (23) and Iraq (2). The sensitivity pattern of P. aeruginosa was alarming in this study, and we found that 67 - 78% of the isolates were resistant to the used antibiotics, including ampicillin, amoxicillin/clavulanic acid, cefazolin, ceftriaxone, cefeprime, ertapenem, ciprofloxacin, and nitrofurantoin. Such high resistance rates to antibiotics in our region can be explained partially by the high rate of antibiotic abuse in the region.
In the present study, 57 (8.9%) isolates of Staphylococcus spp. were obtained from UTI cases. Besides, S. haemolyticus was the most frequent species (3.8%), followed by S. epidermidis (3.02%), S. lentus (1.12%), and S. aureus (0.94%). In contrast, a recent study conducted in Iraq found that 50% of uropathogens were Gram-positive, and the vast majority of them were Staphylococcus spp. (2, 19). Besides, in another study carried out in Iraq on 371 females afflicted with UTIs, Staphylococcus spp. were only found in two (0.5%) cases of UTIs (3). Such discrepancies are difficult to explain and could be due to the differences in sample collection, study design, and patient recruitment. Other frequent Gram-positive isolates reported in the current study were E. faecalis and S. agalactiae, which were responsible for only around 3% of the cases. In the present study, the vast majority of Gram-positive isolates were resistant to benzylpenicillin, oxacillin, clindamycin, erythromycin, and erythromycin accounting for 60% - 100%, while they were highly sensitive to nitrofurantoin, tigecycline, moxifloxacin, and linezolid (100%). Therefore, the use of these antibiotics, especially nitrofurantoin and linezolid, could be considered as empirical treatment for a suspected UTI.
In conclusion, in the present study, E. coli was the most frequent Gram-negative uropathogen causing UTIs among female subjects, followed by K. pneumoniae and P. mirabilis, while Staphylococcus spp. were most predominant among Gram-positive isolates. Ertapenem and imipenem showed to be the most appropriate antibiotics for empirical therapy for Gram-negative uropathogens, whereas linezolid, tigecycline, and nitrofurantoin were for Gram-positive isolates. The continuous monitoring of sensitivity patterns is needed to determine the antibiotics of choice for empirical treatment.