1. Background
Tuberculosis (TB) is a major health problem worldwide. Approximately one-third of the world’s population, approximately two billion people, are infected or at risk of TB. Each year about 9 million people develop active TB, of whom 1.5 to 2 million die of the disease (1). More than 90% of cases of infection and death from TB occur in developing countries, where 75% of the cases belong to the most active group, which is the age group of 15 - 54 years (2).
Although TB is a known disease with defined epidemiology and its treatment principles were demonstrated about 60 years ago, a short-term treatment regimen has been used for it for over a quarter of a century. However, the standard diagnostic method for TB is still the direct observation of Mycobacterium TB or bacterial culture. In addition, a direct bacilli observation method is used in the sputum smear for monitoring and follow-up in response to the treatment. Using this method, which is directly related to the technologist experience and requires lots of time and patience to observe all parts of the smear accurately, may be accompanied by errors. Moreover the Distribution is not uniform in the smear (2).
In 1963, Neopterin (NPT) was separated from the bee larvae and royal jelly bees for the first time, and it was separated from humans later on in 1967 (3). Alveolar macrophages and T helper lymphocytes are the main components of the immune response to mycobacterial infection. NPT (6-D-erythro-trihydroxypropyl pteridine) is one of the indicators of the activity of the immune system in the body, and it has been shown that NPT serum levels increase in infections that stimulate the cellular immune system (4).
NPT is present in T lymphocytes, stimulated by interferon-gamma and to a lesser extent by interferon-alpha and beta, as well as in macrophages, dendritic cells, and endothelial cells, and less in fibroblasts, smooth muscles of the arterial wall, and epithelial cells of the kidney. It is produced from guanosine triphosphate (5, 6).
Increases have been reported in NPT serum levels during a viral infection, such as measles, rubella, varicella, respiratory infections, viral hepatitis, and human immunodeficiency virus (HIV) corresponding to the activity of the virus (7-10). Studies on non-mycobacterial infections have shown that the urinary excretion of NPT is lower than that of viral infection (11). However, the urine NPT level is higher in the pulmonary infection caused by Mycobacterium TB than in bacterial pneumonia and cancer (12-17). Previous studies indicated that the serum adenosine deaminase (ADA) and NPT levels were significantly higher in patients with pulmonary TB than in other patients (13). In addition, the level of these two biomarkers is shown to decrease at the end of two months TB treatment (13). Our previous study did not support the usefulness of serum ADA activity as a diagnostic tool. Cesur et al. (12) found that the serum NPT level was significantly higher in TB patients than in healthy subjects. They, therefore, concluded that serum NPT levels were a useful marker for the diagnosis of pulmonary TB (17).
The necessity of having a non-invasive technique for monitoring response to treatment in patients undergoing pulmonary TB is crucial, especially in cases where the patient cannot give sputum or cannot perform a smear test.
2. Objectives
On the other hand, there is no internal study evaluating the serum NPT level. Therefore, this study was designed to explore the potential of this method. If the results of this study confirm the validity of this method, it can monitor the response to TB treatment in a particular case.
3. Methods
3.1. Method of Execution of the Plan
In this analytical epidemiological study, 34 smear-positive patients diagnosed with pulmonary TB were enrolled. Based on the national protocols (16), the patients had at least two smear-positive sputum samples or a smear-positive sputum sample and clinical symptoms, a smear-positive specimen and radiography indications, or matching pulmonary TB or a smear-positive sputum samplewith positive Mycobacterium TB culture. The exclusion criteria were patients with pulmonary TB combined with pulmonary malignancies or other pulmonary infections, patients immunocompromised for any cause (congenital or acquired), and patients with side effects leading to discontinuation of treatment in the course of study.
Smear-positive pulmonary TB patients hospitalized in the infectious ward of the Razi Hospital (n = 10) and 24 patients referred from the Ahvaz Health Center were recruited for the study. All the patients were informed of the study purpose and procedure, and written consent was taken from them before enrolment. Also, the patients’ demographics were recorded. Three milliliters (ml) of blood were collected in an aseptic condition. The blood samples were sent to the Pasteur laboratory on the same days of blood collection to separate the serum. An enzyme-linked immunosorbent assay (ELISA) was used to measure the serum NPT level via the Brahms ELItest Neopterin kit and the coated plate technique (3). The results were expressed in nano mol per liter. The patients started the anti-TB treatment (including isoniazid (INH), rifampin (RIF), ethambutol (EMB), and pyrazinamide (PZA) drugs), according to the national guidelines, and the serum NPT level was measured at the end of the second and fifth months of the treatment. In addition, according to the national guidelines, sputum smear microscopy (at least two sputum sample (of 5 mL) were collected on consecutive days) was examined at the end of the second and fifth months of the treatment.
3.2. Determination of Sample Size
In order to determine the sample size, a formula was used to compare the means, in which α = 0.05, β = 0.2. Based on the results of previous studies (16), x2 = 32.82, x1 = 46.71, SD1 = 4.9, and SD2 = 4.9, respectively. The prototype size was 24, with a loss of about 20% during the study, and the final sample size was estimated to be 34. Sampling was performed in a non-probabilistic sequential manner. This procedure continued until the final sample size was reached.
3.3. Data Analysis
For data analysis, first, descriptive statistics, including frequency distribution tables, graphs, central indicators, and appropriate dispersion, were used to describe the studied variables. Data analysis was performed using SPSS 24.0 software, and the quantitative data were analyzed using the Kolmogorov-Smirnov test for normalization. Further, repeated measures ANOVA was used to investigate changes in serum NPT mean levels over the treatment period.
A P-value < 0.05 was considered statistically significant.
4. Results
In this study, 34 cases of sputum smear-positive, diagnosed with pulmonary TB were studied, of whom 26 (76.5%) were male. The mean age of the patients was 39.15 ± 14.26 years. Table 1 shows the demographic characteristics of the patients.
| Variables | Values |
|---|---|
| Age, y | 39.15 ± 14.26 |
| Gender | |
| Male | 26 (76.5) |
| Female | 8 (23.5) |
| Marital status | |
| Single | 18 (52.9) |
| Married | 16 (47.1) |
The Demographic Characteristics of Smear-Positive Pulmonary Tuberculosis Patients
In the first stage of sampling, acid-fast bacillus (AFB) sputum was positive in all the patients. At the end of the second month of the standard anti-TB treatment, 25 (73.5%) of the patients had a negative AFB sputum smear, and nine (26.5%) of them had a positive sputum smear. All the patients had a negative AFB sputum smear at the end of the fifth month of the standard anti-TB treatment.
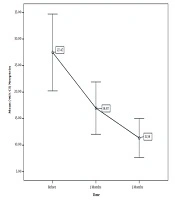
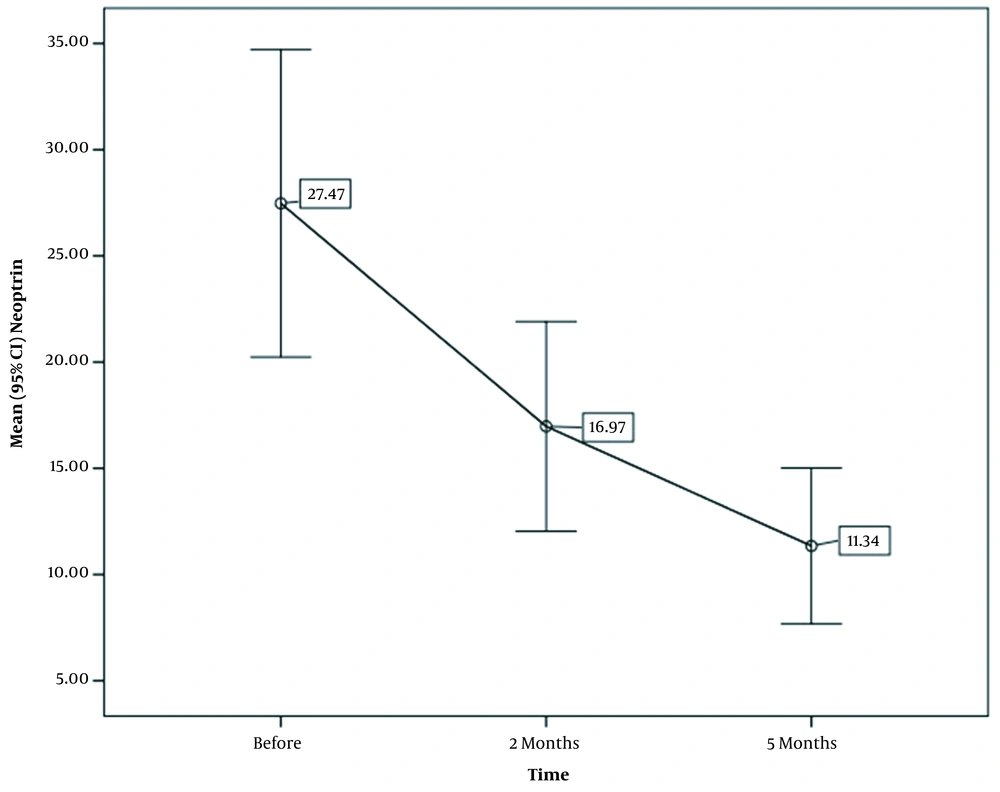
In the study, the serum NPT was measured in three steps. The mean NPT level was measured for the first time at the time of diagnosis and before the treatment, which was 27.47 nmol/L, with a maximum of 104.7 nmol/L and a minimum of 2.9 nmol/L. Two months after the anti-TB treatment, the serum NPT was measured to be 16.97 nmol/L, with a maximum of 60.6 nmol/L and a minimum of 2.1 nmol/L. Finally, the mean NPT level was measured to be 11.3 nmol/L, with a maximum of 46.8 nmol/L and a minimum of 1.1 nmol/L at the end of the fifth month of the anti-TB treatment (Table 2).
| Period | Mean ± SD nmol/L | Minimum nmol/L | Maximum nmol/L |
|---|---|---|---|
| Before the treatment | 27.47 ± 20.7 | 2.9 | 104.7 |
| At the end of the second month | 16.97 ± 14.1 | 2.1 | 60.6 |
| At the end of the fifth month | 11.3 ± 10.5 | 1.1 | 46.8 |
Serum Levels of Neopterin in Smear-Positive Pulmonary Tuberculosis Patients During the 5 Month Therapy Follow-up
The serum NPT level in patients with pulmonary TB was examined before the treatment, in the second month of the treatment, in the fifth month of the treatment, at the end of the second month, and at the end of the fifth month (Figure 1).
The mean serum NPT at the end of the second month of the anti-TB treatment in AFB-positive patients (23.89 ± 9.88 nmol/L) was higher than the mean serum NPT in negative AFB cases (20.6 ± 4.89 nmol/L), although not statistically significant (P > 0.001). Also, the NPT level decreased with increasing age at the end of the fifth month of the treatment compared to the pre-treatment, although not statistically significant (P > 0.001). In addition, the decrease of the NPT level in women was not statistically significant at the end of the fifth month of the treatment compared to the pre-treatment (P < 0.001).
5. Discussion
TB is one of the major diseases and health problems worldwide. Nearly two billion people in the world are infected or at risk of TB (1). Considering that the diagnosis of TB is of particular importance in patients’ public health and well-being, diagnostic and follow-up methods are crucial. At present, the discovery of new techniques with high sensitivity and specificity to detect and follow up with TB patients is of interest to researchers worldwide.
Considering that many patients cannot give a sample of sputum to follow the response of treatment, we here discussed the serial measurements of the serum NPT level to monitor the patient during treatment.
In our study, the mean age of the subjects was 39.15 ± 14.26 years, with 76.5% of them being male and the rest being female. The highest frequency was in the age group of 21-40 years (53%). In the study of Altunoglu et al. (13), the mean age of patients was 43.41 ± 11.48 years. the participants in the current study were younger than other studies.
The age difference is apparently due to the endemic nature of patients in Khuzestan Province and the prevalence of TB among them at an earlier age. The difference in life expectancy in different countries also affects the age of patients.
In the present study, concerning the correlation between age and the NPT serum level, the serum NPT level was lower at the end of the fifth month of the treatment with increasing age, although not significant. This result is consistent with the results of Aminiafshar et al. (9), indicating that the decrease in NPT was not related to the age and sex of patients, and no discussion has been found in other studies on the correlation between age and sex with the reduced NPT.
In addition, it was found in the present study that NPT was further reduced in women at the end of the fifth month of the treatment compared to the pre-treatment, although not statistically significant. No other studies found similar results.
In our study, the mean serum NPT level was 27.47 ± 20.7, 16.97 ± 14.1, and 11.34 ± 10.5 before the treatment, at the end of the second month of the treatment, and at the end of the fifth month of the treatment, respectively. In Altunoglu et al. study (13), the mean serum NPT was 46.71 ± 10.26 after two months of treatment was 32.82 ± 10.52 and before treatment and at the end of the second month was 3.72 ± 1.46 and 1.49 ± 0.69, respectively. In Cesur et al. study (12) in Ankara, NPT was 23.74 ± 21.8 nmol/L in patients before treatment (12). Turgut et al. (16) reported NPT to be 69.54 ± 29.42, 54.16 ± 19.25, 25.6 ± 9.12, and 12.89 ± 6.8 before treatment and two, four, and five months after treatment, respectively. In Immanuel et al. study (17), the serum NPT level was 39.9, 29.5, and 16 before treatment, one month after treatment, and at the end of treatment, respectively, with a reduction rate of 37% during treatment and 66% at the end of treatment.
The difference in serum NPT levels can be due to different methods used in the studies, such as different laboratory kits or various severities of the disease, as mentioned in some studies (15, 16). The NPT level was also affected by the pulmonary disease severity and duration of symptoms, given that most of our samplings were taken from outpatients. Therefore, our patients may have a lower NPT level, and also, the nutrition affects the serum NPT level (15). These factors can also justify the difference in the NPT levelin different studies. However, the definitive cause of lower NPT levels is unclear, and further studies with larger sample size and the differentiation of outpatients and hospitalized patients are recommended. In general, the studies mentioned above have not taken into account the absolute number of NPT levels. However, the NPT downward trend has mostly been considered, and thus it does not affect the results negatively.
5.1. Conclusions
According to the national protocol for TB treatment, the sputum smear is still essential in patients’ follow-up. However, NPT serum level measurements can be used in patients with pulmonary TB under anti-TB treatment to monitor the response to treatment and for patients’ follow-up.