1. Background
Down syndrome (DS) is the most common (1) and well-known chromosomal disorder, and it is the most common genetic cause of moderate mental retardation (2). Its prevalence increases with increasing maternal age (3). Although its prevalence at birth varies in different societies, it is generally about 1 in 800 to 1 in 1000 per live births (2). The prevalence of DS in Iran is lower than the global average, and it is 0.9 per 1000 live births (4), of which 77.5% is trisomy, 18% is mosaicism, and 4.5% is chromosomal translocation (5).
Like other chromosomal abnormalities, DS can be diagnosed before birth using screening methods that are divided into the first-trimester and second-trimester screening tests.
First-trimester screening test is a combined screening that involves a combination of Nuchal Translucency (NT) ultrasound and two serum markers in the mother's blood. As legal abortion is allowed up to 16 weeks of pregnancy in Iran, the only applicable method that can be used to screen DS fetus prenatally in Iran is combined test, which is officially integrated into the healthcare system of Iran since 2012 and is recommended for every pregnant woman.
Today, due to the higher costs of living of children with DS due to the associated diseases and disabilities, DS fetus screening is common before birth to prevent them from being born. As healthcare costs are increasing, healthcare systems, health insurance providers, and patients are interested in controlling them (6). Hence, every intervention in the health system should be evaluated using health technology assessment (HTA), which is a systematic way to analyze the outcomes of various interventions; and economic evaluation is an important part of it (7).
The economic evaluation technique used in this study was cost-benefit analysis (CBA) method. The CBA method assumes that the health outcome is the same in different methods and compares them only in terms of cost. In cases where it is necessary to choose between different alternatives, the method with the highest net monetary benefit (NMB) is recommended (8).
2. Objectives
This study aimed to evaluate the economic aspects of combined first trimester screening in Iran.
3. Methods
The research environment of this cross-sectional and descriptive study was health centers in Tehran, Iran. The study population included all pregnant women referring to the health centers for prenatal care. The study was performed from a societal perspective. The study was approved by Iran University of Medical Sciences (code: IR.IUMS.FMD.REC.1398.010).
In this retrospective cohort study, the information of 1,547 pregnant women referred to Tehran health centers was obtained from integrated health system (IHS) entitled ‘SIB’.
3.1. Modeling
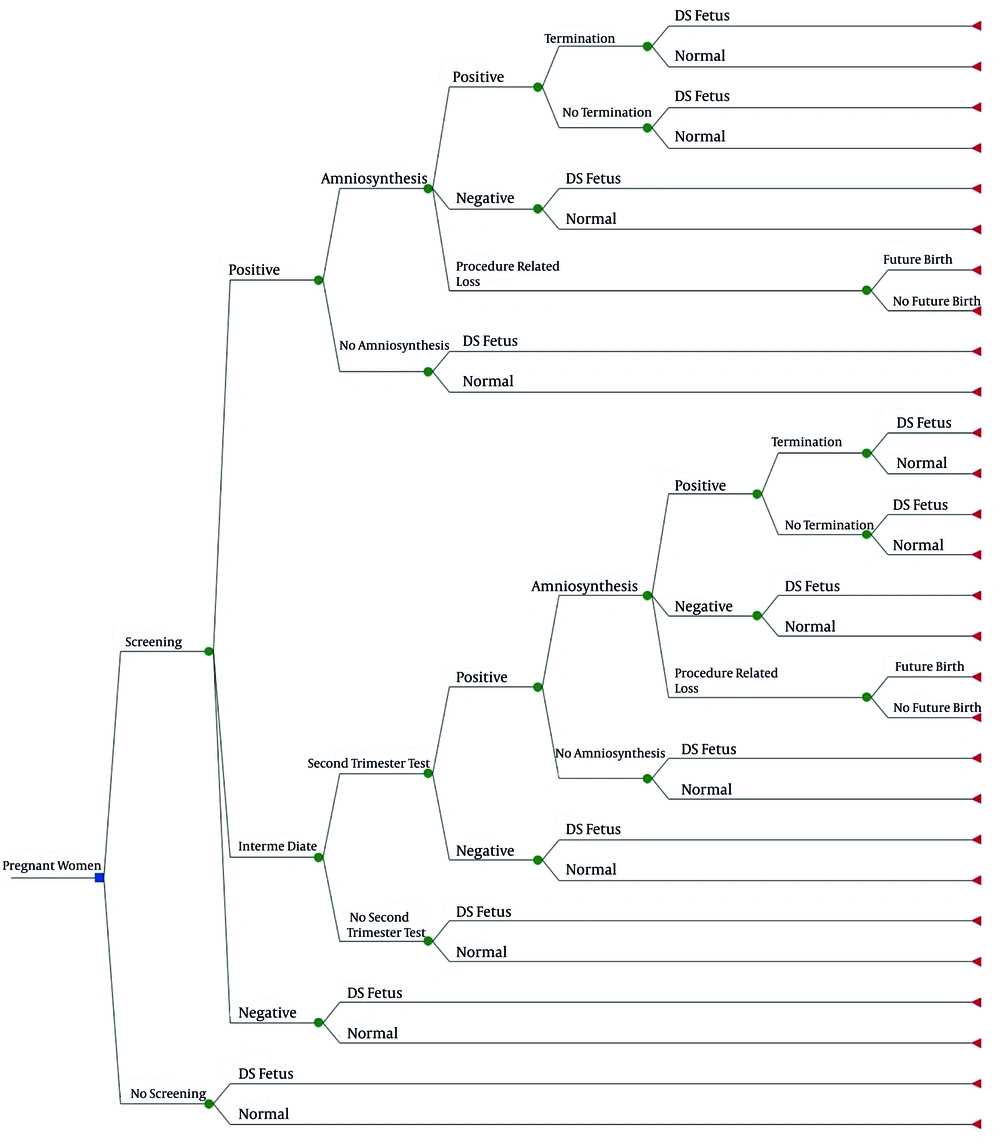
In the economic evaluation and decision-analytic model, we compared two strategies: (1) DS screening, versus (2) no screening. Some methods can be performed in the second trimester for DS screening. However, since legal abortion is allowed up to 16 weeks of pregnancy in Iran, it is not possible to use the second-trimester tests because they are performed after this period. Therefore, comparing first-trimester screening with the second-trimester screening tests does not have a practical outcome. Hence, in this study, we compared screening with no screening. We calculated the cost of averting a DS fetus and compared it with the cost of not identifying an affected fetus, which is equal to the incremental living cost of a child with DS. Incremental living cost of a DS baby is the difference between the cost of living for a DS child and a healthy child. Because screening prevents imposing additional costs of a DS child to the community, we considered the incremental living cost of a DS baby as the benefit by preventing the birth of an affected baby. We used a decision tree model in our study (Figure 1).
According to this model, in the screening arm, people who receive a positive result in the first stage may or may not accept amniocentesis. If amniocentesis is performed, the consequences may be a healthy fetus (false positive) or a DS fetus (true positive). There is another possibility, that is, the death of a healthy fetus as a result of amniocentesis or procedure-related loss (PRL). But if the person does not perform amniocentesis, a healthy (false positive) or an affected infant (true positive) may be born. In the case of patients diagnosed with amniocentesis, abortion therapy is performed; however, if the abortion is not accepted by the parents, an affected baby (true positive) might be born. The birth of a healthy baby following the diagnosis of affected fetus is due to the fact that the sensitivity and specificity of the diagnostic test is not 100% (9).
For the first trimester tests, a third answer called ‘unknown’ or ‘intermediate’ is possible. If the answer is ‘intermediate’, the second-trimester test is performed, and the decision is made based on it.
The time horizon of the model was 15 years, equal to the average life expectancy of a DS child. Although there is no data on the average life expectancy of DS patients in Iran, we assumed it about 15 years according to the Yang Q (2002) study, which examined the difference in life expectancy of patients in the United States from 1983 to 1997, and estimated that the median life expectancy of non-American races in 1997 is about 15 years (10).
Since synthesis-based estimates were used in our study, some estimates were extracted from the cohort study and the others obtained from various articles. The probability of DS prevalence, probability of termination after diagnosis, probability of PRL, probability of future birth after PRL, utility of having a DS baby, utility of future birth after PRL, and utility of no future birth after PRL were extracted from the articles, and the other parameters were obtained from our study (Table 1).
| Parameters | Values | Source |
|---|---|---|
| Probabilities | ||
| Probability of DS prevalence | 0.0011 | (2) |
| Probability of termination after diagnosis | 0.99 | (11) |
| Probability of PRL | 0.005 | (11, 12) |
| Probability of future birth after PRL | 0.409 | (13) |
| Probability of accepting DS screening | 0.0046 | Our study |
| Probability of having ‘intermediate’ result in first-trimester screening | 0.0057 | Our study |
| Utilities | ||
| Utility of having a DS baby | 0.7 | (13) |
| Utility of future birth after miscarriage | 0.87 | (13) |
| Utility of no future birth after miscarriage | 0.7 | (13) |
| Costs (USD) | ||
| Cost of first-trimester test | 111 | Our study |
| Cost of second-trimester test | 117 | Our study |
| Cost of amniocentesis | 465 | Our study |
| Cost of termination | 167 | Our study |
| Benefits (USD) | ||
| Cost of heart problems | 1000 | (14) and our study |
| Cost of speech problems | 2380 | (15) and our study |
| Cost of hearing problems | 2000 | (16) and our study |
| Cost of vision problems | 333 | (15) and our study |
| Cost of other problems | 2380 |
Input Parameters of the Study
3.2. Cost Estimations
To calculate the cost of identifying one DS fetus, the direct and indirect costs of DS screening in 2019 were calculated from a societal perspective, which included costs of testing, specialist visit, services provided by health centers, traveling, and mother’s absence from work.
3.3. Benefits Estimation
To calculate the benefits of screening, the incremental living cost of a DS child was calculated; such costs could be prevented through diagnosing before birth.
To calculate the incremental cost of living of a DS child, the prevalence of various DS disabilities was multiplied by the cost required to reduce disability. The prevalence of heart disorders in these children is 50%, many of whom require surgery (14). Moreover, in DS children, the prevalence of hearing problems is about 75%, conductive disorders are about 83% (17), cataracts is about 3% (17), visual problems is up to 60% (17), thyroid disease is about 4 - 18% (16), and speech disorders with varying degrees is about 97.8% (15). Besides, adults with aging are at increased risk for Alzheimer's disease (18), and the onset age of dementia in people with DS is 56 years (19). Almost all people over the age of 40 with DS have sufficient neuropathology for diagnosis of Alzheimer's disease (20).
To calculate the cost of heart disease in DS babies, the direct and indirect cost of treatment is multiplied by the prevalence of heart problems in these children (50%) and the proportion use of this service in Iran (32%), [(open-heart surgery + 20 times referred to a cardiac surgery specialist + 20 days absence from work + travel expense) × 50% × 32%] (21).
To calculate the cost of managing speech disorders in DS children, it was assumed that 50% of these children use speech therapy services for two years (two sessions per week). So, the cost of one session of speech therapy (78,000 Tomans; about 5 USD), plus travel costs and absence from work were multiplied by the number of weeks in a year (82), the number of sessions per week, the proportion of use of this service in Iran (50%), and the prevalence of speech disorder (97.8%) (15).
To calculate the cost of hearing problems, the costs of an specialist visit, hearing tests, hearing aids, absence from work, and travel costs were multiplied by the prevalence of hearing problems (75%) in DS children (17); since the service must be repeated every 5 years, this amount was multiplied by 3 according to the average life expectancy of these people (15 years).
To calculate the cost of visual impairment in DS children, the direct and indirect costs were multiplied by the prevalence of cataract (3%) (17) and the maximum probability of patients needing glasses (60%) (17). The cost of providing the glasses was multiplied by 7, because it is necessary to repurchase the glasses every 2 years.
For other problems that are less common in these people, 10 million Tomans (about 667 USD) was considered. However, families rarely spend money on children with DS in low- and middle-income countries (LMICs), so that some of these children even do not go to school.
Considering the average life expectancy of patients, the possibility of Alzheimer's disease and its costs were not calculated.
3.4. Cost-Benefit Analysis (CBA)
Regarding the costs and benefits estimation of strategies, CBA was performed. CBA is usually performed by net present value (NPV) index. The following formula was used to obtain the NPV of DS screening:
In this formula, benefit and cost represent incremental benefits and cost of DS screening versus no screening, ‘r’ represents the discount rate, and ‘t’ represents the years of study (22). Given that our study lasted less than one year, ‘r’ was equal to zero, and ‘t’ was considered equal to one. All data analyses were conducted using TreeAge 2011 and Excel 2016 software.
4. Results
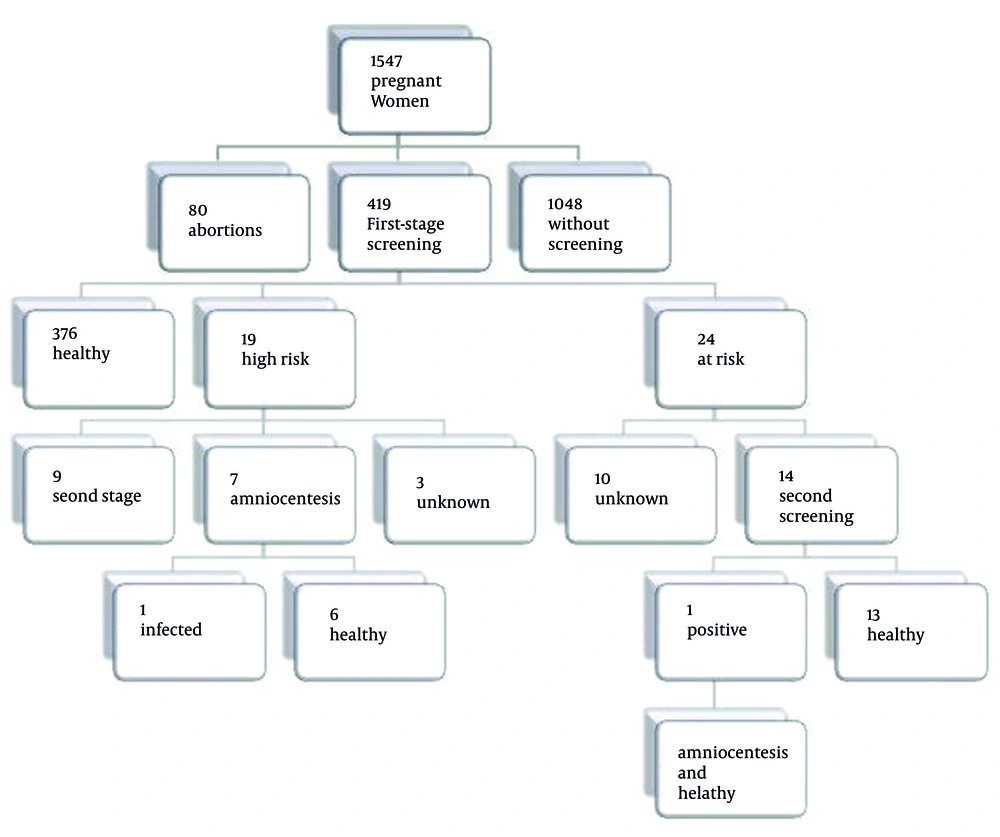
Our cohort study showed that 30% of pregnant women referring to Tehran health centers accept the first-trimester screening, among whom 4.6 had a positive (abnormal) result, 5.7 had an intermediate result, and other 89.7% had a normal test (Figure 2).
4.1. Costs
The screening cost of the first stage was 467,000 Tomans (about 31 USD). The cost of the second phase of screening, including the total costs of test, specialist visit, travel, and mother's absence from work was 491,000 Tomans (about 33 USD).
Taking into account the direct and indirect costs of DS screening, the total cost of screening to diagnose a case of DS was equal to 611 million Tomans (about 25,000 USD).
4.2. Benefits
The cost of heart problems in children with DS was estimated about 4,000,000 Tomans (about 267 USD), speech therapy about 10,000,000 Tomans (about 670 USD), hearing problems about 8,400,000 Tomans (about 560 USD), and visual problems about 1,400,000 (about 94 USD). Also, 10 million Tomans (about 670 USD) was considered for the miscellaneous costs of these children.
Taking into account the direct and indirect costs, the incremental living cost of children with DS in Iran was an average of 34,000,000 Tomans (about 2,270 USD).
4.3. Cost-Benefit Analysis
The total cost of screening for diagnosing a DS case was equal to 611 million Tomans (around 25,000 USD), and the incremental living cost of children with DS was 34,000,000 Tomans (about 2,270 USD); hence, the difference between the benefits of screening and the cost of screening to find an affected child (NPV) was negative (about -136,000 USD).
5. Discussion
This economic evaluation study aimed to evaluate the costs and benefits of DS screening in Iran from the societal perspective. Various perspectives can be used in conducting economic evaluation studies, including the societal perspective and healthcare perspective (23). In this study, we used the social perspective, which is the classic approach to economic evaluation. In the social perspective, all direct and indirect costs and benefits are taken into account, regardless of who incurs or benefits from it (24). It is recommended to perform CBA method from the societal perspective because both taking care of a DS child and the loss of a healthy fetus following diagnostic tests are all societal burden (25).
The cost of screening for DS in Iran was 611 million Tomans (about 25,000 USD), which is different from other countries. For example, this amount is about 35,851 USD in the United States (2005) (26) and 176,000 Euros in the Netherlands (2008) (27). Thus, the cost of finding a case of DS varies from country to country due to the differences in prices and the variety of services offered in different countries.
In the present study, the benefit of the screening or the incremental living cost of children with DS was about 34 million Tomans (about 1400 USD), which is different from other studies. For example, the incremental cost of living of an affected child was 471,000 USD in 2013 in Australia (28). Also, the average total cost of healthcare for people with DS in Australia was 4,209 USD per year, with a median of 1,701 USD in 2011. The skewed distribution of these costs is because half of the costs are spent for only 10% of people. This study has shown that median costs decrease rapidly after the first and second years of life (29). A study in the United States also found that the cost of healthcare for affected children was 13 times that of normal children (30), but this calculation was made only by examining children with health insurance, that is a sign of well-being in the United States. Another study in Denmark found that the cost of adolescents with DS is only 1.7 times higher than healthy adolescents (31). Part of this difference is due to the facilities and services available in different countries for DS children. In this study, we considered the costs of heart surgery, speech therapy, audiometry and hearing aids, glasses, cataract surgery, and other costs.
The NPV of DS screening was negative, and it was equal to 577,000,000 Tomans (about 23,000 USD). In CBA method, a strategy is accepted when its benefit is more than the costs.
Our study had some limitations. We did not calculate the psychological costs of having a DS baby, and either the psychological cost of awareness of the positive screening results, or missing the baby because of diagnostic procedures. Also, the cost of the impossibility of pregnancy in a specific period after termination of pregnancy was not included in the calculations.
5.1. Conclusion
The result of this study showed the screening tests for DS needs revision and consideration in Iran, and due to the high cost of screening, coverage with insurance organization is necessary.