1. Context
Nowadays, high-energy photons are applied for the treatment of deep tumors due to their skin-sparing effect and higher penetration depth. One of the complications with high-energy photon therapy is secondary electrons generating from the interaction of photons with heavy materials in the beam path, which shifts the maximum dose depth toward the surface and raises the surface dose (1-3).
Several studies have investigated various factors affecting electron contamination (2-5). In this regard, Petti et al. (3) reported that the flattening filter and also the monitor chamber located in the linear accelerator’s head have the most significant contribution in electron contamination, and 17% of the secondary electrons were produced in the air column after jaws. Also, at larger source skin distances (SSDs), electron contamination in the air was increased (3). It has been shown that for absorbing or scattering most of the secondary electrons in the beam, it is appropriate to have an air gap of 15 to 20 cm between the absorber and the skin before entering the tissue (6). For higher energies, electron contribution is due to the beam flattening filter and photon collimators (7). Klevenhagen (4) concluded that by decreasing the treatment field size, the contaminating electrons are scattered; thus, the surface dose will be reduced. Lopez Medina et al. (5) measured electron contamination and showed that it depends on photon energy, treatment field size, beam shaper, and SSD. Also, they reported the greater dependence of electron contamination on field size, SSD, and depth for 18 MV energy than 6 MV (5). Therefore, we reviewed different articles about sources of electron contamination of photon beams applied for radiation therapy and methods of measuring the contribution of contaminating electrons in dose distributions to provide more knowledge about the clinical beams' energy, which helps to advance the accurate treatment planning systems capable of considering electron contamination in the radiation therapy calculations.
2. Methods
This scoping review was performed following the PRISMA-ScR guidelines (8).
2.1. Search Strategy
This study was performed to review the measurements and calculations of electron contamination for radiotherapy photon mode. We searched five major indexing databases, including PubMed, Scopus, Embase, ISI web of science, and Cochrane central, using keywords of electron contamination, electron contamination AND measurement, electron contamination AND simulation, and electron contamination AND reduction until Dec 2020. No language restriction was performed. Then, articles not relevant to electron contamination and not relevant to radiotherapy were excluded. Abstracts were screened for relevance to the clinical outcomes of the procedures.
2.2. Inclusion and Exclusion Criteria
Title and abstract screening of initial selected studies for inclusion or exclusion criteria was performed independently by the reviewers (NCH and FR). Any disagreement between two reviewers was resolved by either discussion or the help of a third reviewer (MT). Only original articles were eligible if they provided all of the following characteristics: (A) electron contamination in radiotherapy; (B) dosimetry and simulation methods; and (C) electron contamination reduction techniques. Studies were excluded if they were: (A) narrative or systematic reviews, letter to editorial, and guideline; (B) not relevant to electron contamination; and (C) not relevant to radiotherapy.
3. Results
3.1. Summary of Findings
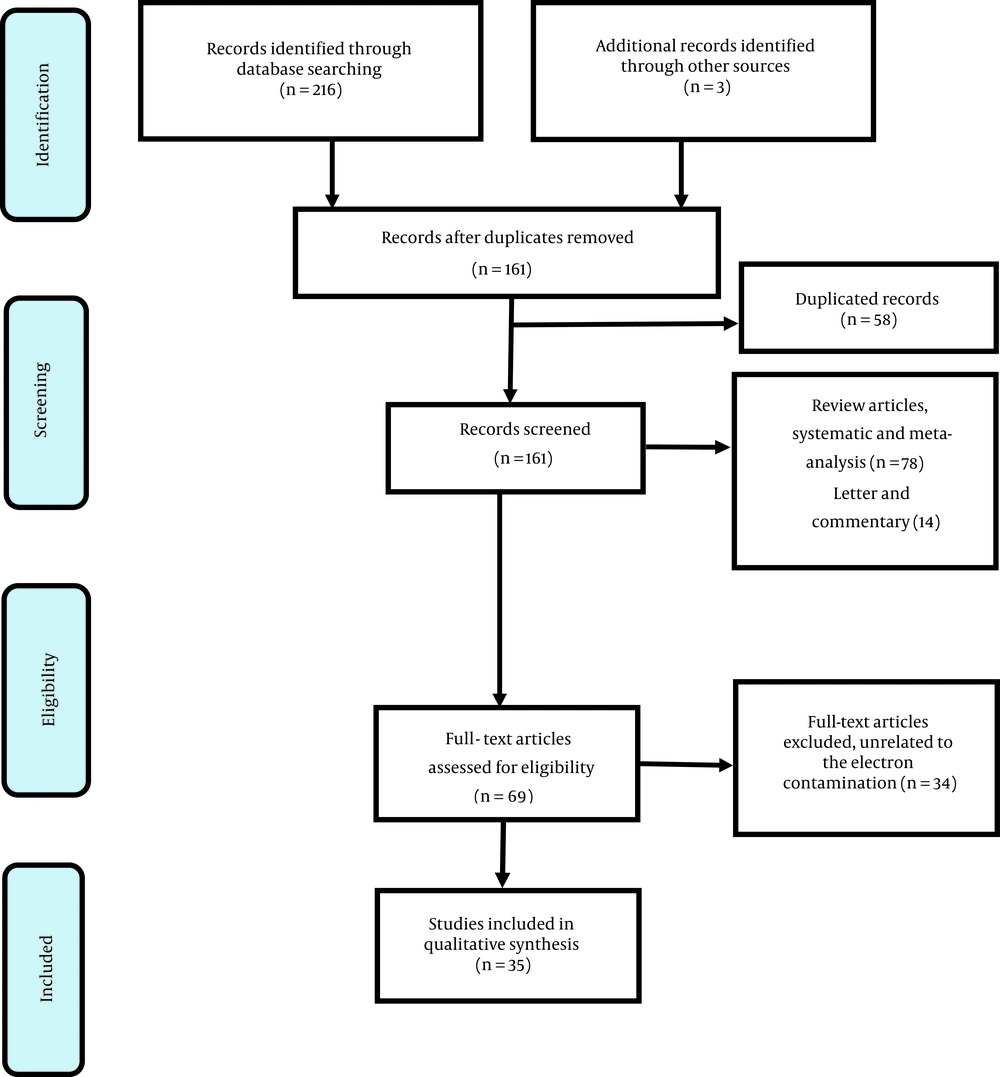
Through searching, 219 records were recognized; 161 records remained after removing 58 duplicated articles. Also, 92 records were excluded due to not being related to radiotherapy. In the next stage, 69 papers were screened. Finally, 35 studies remained for full review based on reasons in Figure 1. Eleven articles had reported the theory of electron contamination.
3.2. Techniques to Estimate Electron Contamination
Some studies had used methods as dosimetry measurements or Monte Carlo simulations to assess electron contamination, which are discussed in this article.
3.3. Theory
As it is not possible to separate the dose contributions of electrons and photons, generally, accurate measuring of the dose in the buildup region is difficult (9). The most widely used theories for determining these doses are the multiple scattering theory (MST) proposed by Rossi and Greisen in 1941, and the theory of Eyges in 1948, known as the Fermi-Eyges theory. According to these theories, calculations are done based on the electron transport model, which includes electron production by photons, transport, and several scatters of the produced electrons (10-13).
Johns in 1951 and Yorke in 1985 discussed that the air is a source of electron contamination and a scattering medium for the electrons (14, 15). Bertilsson (16) illustrated this by cavity theory, taking the transition effects on the fluence of the electrons into account.
Higgins et al. (17) presented a method for calculating the relative contribution of contaminating electrons in skin dose for Co-60 gamma photons. They applied the Klein-Nishina differential scattering to assess the initial energy and the number of scattered electrons into a detector direction. Also, a Gaussian estimation was employed to determine the surface distribution of contaminating electrons. It was found that for SSDs > 80 cm, the relative contribution of contaminating electrons produced in the air was even greater than 50% of the total measured skin dose (17). Ahnesjo et al. (18) in 1992 showed that the fluence of contamination from charged particles at the surface can be approximated with the Gaussian model. Beauvais et al. (19) analyzed the surface dose under a wide range of energies (4, 6, 12, 18, and 25 MV) and developed an approach to extrapolate the contribution of electron contamination in depth doses. Bjarngard et al. (20) in 1995 improved this method using a model of the dose based on scattering photons generated in the surface of phantom and parameterization of the dose from electron contamination using the analytical function. Zhu and Palta (21) developed this method using the percentage depth dose data. Yang et al. (22) developed a Gaussian planar source located on the upper jaws' surface to model the contaminating electrons. They concluded that the proposed source size depends on the applied treatment field size (22).
Ulmer et al. (23) stated the flattening filter, primary collimator, monitor chamber, air, and jaws as the sources of producing contamination. They showed the dependency of contaminating electrons fluence to the jaw positions for small fields. Also, they concluded that the contaminating electrons can be represented with the convolution of a Gaussian distribution (23). In this regard, Sadrollahi et al. (24) stated the significant contribution of electron contamination in the surface dose of flattened photon beams. Also, they showed the reduction of the penumbra, surface dose, and the mean energy of photon beams when removing the flattening filter (24).
Gonzalez et al. (25) developed a general model to define the sources of contaminating electrons for any linacs when utilizing photon mode. They proposed a two-source model, in which the electrons produced in the head of the treatment system and also the distance between its head and the patient’s surface. Their results showed that the proposed model for calculation of electron contamination is properly capable of describing the absorbed dose in a water phantom for all considered treatment field sizes (25).
3.4. Dosimetry Results
Reviewed studies about measuring electron contamination using dosimetric methods are summarized in Table 1. Accordingly, thermoluminescent dosimeter (TLD), LiF dosimeter, parallel plate chamber, diode, and optically stimulated luminescent dosimeters (OSLD) have been used for measuring electron contamination. The results indicated the reduction of electron contamination by increasing the source distance to the point of measurement by the dosimeter. Also, electron contamination rises with the energy of the photon beam and at the edges of the treatment field (Table 1).
| Year | Authors | System Equipment | Photon Beam Energy, MV | Type of Dosimeter | Contamination Range | SDD, cm |
|---|---|---|---|---|---|---|
| 1999 | Hounsell and Wilkinson (26) | Elekta (SL15) | 8; 4; 20 | Parallel plate chamber and poly methyl methacrylate (PMMA) sheets | The average energy of the contaminating electrons was maximum for 20 MV photon beam. | 70 - 140 |
| 2001 | Butson et al. (27) | Varian (2100C) | 6 | Attix parallel plate ionization chamber and radiochromic film | Electron contamination originated from blocking tray can affect dose distribution in radiotherapy. The amount of this electron contamination was maximum for 40 × 40cm2 field size. | 100 |
| 2010 | Kumar et al. (28) | 60Co | 1.17, 1.33 MeV | TLD badge and LiF crystals | The electron contamination for 60Co photons originates mainly from source shielding and can be decreased by an increase in the distance between the source and the dosimeter. | 50 - 100 |
| 2006 | Edwards et al. (29) | Varian (2100CD) | 6 | Scanditronix PFD diode and water phantom | Electron contribution to the total dose depends on field size and changed from about 8% on the central axis of the smallest field to about 76% at 10 cm outside the edge of the largest field | 100 |
| 2016 | Narayanasamy et al. (30) | Varian (23EX) | 6 | Optically stimulated luminescent dosimeters (OSLD) | Ion chamber readings when using 5, 10, and 15mm bolus were about 31%, 22%, and 10% of the readings for open beam. | 100 |
Review of Studies with Electron Contamination Dosimetry Results
3.5. Simulation Techniques Results
The results of the reviewed articles related to electron contamination calculating using simulation methods are shown in Table 2. The simulation softwares, including MCNPX, BEAMnrc user code, MCSIM, EGSnrc, MC code PENELOPE, MCRAD, and GEANT4, were applied in reviewed studies. The results of investigated studies revealed a good agreement between simulation techniques’ findings and the measurements. Thus, the results indicated an increase in electron contamination when using a flattering filter, by increasing field size or photon energy, and in the presence of a prosthesis in the patient's body (Table 2).
| Year | Authors | System Equipment | Energy | Simulation code | Important Findings | SCD, cm |
|---|---|---|---|---|---|---|
| 2001 | Malataras et al. (31) | Saturne-25 and -41 GE-Medical systems | 6-12-15-23 MV | MCRAD | The contaminating electron contribution on the surface dose of photon beam with energies of 6, 12, 15, and 23 MV was calculated to be 16%, 16%, 6%, and 17%, respectively. | 100 |
| 2004 | Yang et al. (22) | Siemens PRIMUS | 6, 10, and 18 MV | BEAM and MCSIM | A clinical photon beam was considered to be consist of a primary photon source, an extra-focal scatter photon source, and a planar contaminant electron source. The developed model showed the proportionality of the electron contamination source to the field size. However, for clinically used photon beams, the relative contaminant electron source intensity can be considered constant. | 100 |
| 2011 | Allahverdi et al. (2) | Elekta (SL-25) | 18 MV | MCNPX And also, dosimetry with PTW-MP2 scanner with an ionization chamber (0.6 CC) at the water phantom | The electron contamination contribution in surface dose ranged from 6.1% for 5 × 5 cm2 to 38.8% for 40 × 40 cm2 field sizes. Also, contaminating electron contribution at depth dose was 0.9% up to 5.77% for these field sizes, respectively. Surface dose for the field with tray showed an increase of 2.3%, 7.3%, and 21.4% for 10 × 10, 20 × 20, and 40 × 40 cm2 field sizes, respectively compared with the standard field without any accessories. While, for the fields with the wedge, the electron contamination decreased. | 100 |
| 2009 | Sikora and Alber (32) | ELEKTA SLi | 6 and 15 MV | BEAMnrc | The presented Gaussian source model for contaminating electrons, which was considered to be located at the base of the flattening filter, showed high accuracy for 3D dose evaluation of the surface dose in CT-based patient geometry. | 100 |
| 2010 | Lye et al. (33) | lead cutouts used in kilovoltage | 150 - 300 KVP | EGSnrc | The dose at the edges of the field compared with the central dose was doubled due to electron contamination for a 150 kVp beam and three times for a 300 kVp beam. | 30 |
| 2011 | Asuni et al. (34) | Linac head together with the COMPASS transmission detector | 6 MV | BEAMnrc | The contribution of contaminant electrons to dose in the buildup region increases with increasing field size. These electrons are less energetic and have a larger angular spread compared with contaminant electrons in an open field at shorter SSDs. | 70, 80, 90, and 100 |
| 2013 | Bahreyni Toossi et al. (35) | Siemens PRIMUS linac | 15 MV | MCNPX | The presence of a prosthesis in a phantom can increase both electron and neutron contaminations. The dose increase factor for neutron contamination is more than that for electron contamination. | 100 |
| 2015 | Gonzalez et al. (25) | Clinical linacs | 6; 5; 18 MV | MC code PENELOPE | Developing a general model to define the sources of contaminating electrons for any linacs and proposing a two-source model to consider the electrons produced in the head of the treatment system and also the distance between its head and the patient’s surface. | 100 |
| 2015 | Seif and Bayatiani (36) | Varian (2100 C/D) linac | 6 MV | MCNPX | The contribution of the secondary contamination electrons on the surface dose was 6% for 5 × 5 cm2 to 27% for 40 × 40 cm2, respectively | 100 |
| 2016 | Jagtap et al. (37) | Cobalt-60 | 1.17; 1.33 MV | BEAMnrc | At source skin distance (SSD) of 60 cm for a large field size of 30 × 30 cm2, the relative surface dose increases nearly 2.5 times to that of 10 × 10 cm2 field size. However, at an SSD of 80 cm, it increases nearly 2.1 times of the field size of 10 × 10 cm2. | 60 and 80 |
| 2016 | Yani et al. (38) | Varian Trilogy Clinac iX | 6 MV | EGSnrc | The amount of electron contamination ranges from 0.3 to 0.5% for 6 MV photon beams. This corresponds to 1.4 and 3.9% surface dose, respectively, depending on field sizes. | 100 |
| 2019 | Chegeni et al. (39) | Varian (2100 C/D) linac | 6 MV | BEAMnrc | Replacing the air column with helium decreased the surface dose by about 10%. However, the height of the air column performs as a filter for low-energy electrons. Also, the lead filter decreased electron contamination by about 3.5%, while light elements, such as Aluminum and PMMA had no significant effect on electron contamination decrease. | 100 |
| 2017 | Chegeni et al. (40) | Varian (2100 C/D) linac | 6 MV | Primo | The maximum dose occurred at the depth of 1.3 cm in the presence of electron contamination, while this depth increased to 2 cm with removing electron contamination. | 100 |
| 2019 | Shukla et al. (41) | Co-60 | 1.17; 1.33 MV | BEAMnrc | The electron contamination removal efficiency of filters with a low atomic numbers was low. The tin, copper, and nickel were found effective filters for removing nearly 38% of contaminant electrons. The lead filter was as effective as tin, while the high-energy electrons emitted from the lead filter increases the dose significantly at 3.0 to 4.0 mm depth. | 80 |
| 2019 | Sadrollahi et al. (24) | Siemens Artiste 6 MV and FFF 7 MV beams | 6 and 7 MV | Geant4-10-03 | Removing the flattening filter from the linac head increases the contaminant electron fluence by a factor of 3.94 for a 5 × 5 cm2 field size. Also, it increases the dose rate and decreases the out-of-field dose, while increased skin dose and deteriorated flatness of lateral dose profile. | 100 |
| 2020 | Anam et al. (42) | Elekta linear accelerator | 6 MV | BEAMnrc | Electron contamination increased from 3.7 to 20.9% with increasing field size from 5 × 5 to 40 × 40 cm2. Electron contamination was mainly produced in the air column between the linac head and the phantom. | 90 |
Review of Studies with Electron Contamination Results Using Monte Carlo Simulation
4. Discussion
High-energy photons used in radiation therapy always generate contaminating electrons due to inelastic scattering interactions with materials placed in the beam path, such as monitoring unit ion chambers, flattening filters, beam collimators, wedges, compensator blocks, etc. (43). Also, electron contamination can be produced from the interaction of high-energy photons with the air molecules between the radiation source and the patient body surface (36, 43). Because the number of contaminating electrons can be different for each treatment system, the knowledge of the contaminating electron’s characteristics and calculation of electron contamination are important for clinical dosimetry. Surface dose increment due to electron contamination can damage patients’ skin. Hence, determining the electron contamination for photon beams is critical for proper commissioning and checking the treatment planning system calculations, especially in the build-up region (36). In this regard, different researchers have conducted studies to determine sources of electron contamination or measuring or calculating it for photon beams in the therapeutic energy ranges. We conducted this study to review the techniques and methods for the measurement and calculation of electron contamination for radiotherapy photon mode. Based on our selection criteria, this narrative review consisted of 35 selected articles among many studies. Generally, dosimetry-based studies using TLD, LiF, parallel plate chamber, diode, and OSLD to measure electron contamination, and simulation studies using MCNP, BEAMnrc, MCRAD, PENELOPE, EGSnrc, and GEANT4 softwares to calculate the electron contamination, were reviewed.
The results of studies that had assessed the electron contamination sources in medical linear accelerators, indicated the flattening filters and air below the collimators, as two main sources of contaminating electrons for large radiation field sizes (24, 36, 42). Also, according to the results of reviewed article, the amount of electron contamination depends on the radiation field size (2, 24, 29, 34, 36, 37, 42) because for larger field sizes, the primary photons interact with more surface area of the collimator and air column between the linac head and the patient plane, which produces contaminating electrons and increases the surface dose (37).
Also, based on the results of reviewed articles (29, 36), electron contamination contribution in different MV photon beams to the total dose and the surface dose varied from 8% to 76% from the central axis to the edges of the radiation field and outside it. In this regard, Edwards et al. (29) revealed that the measured dose by a diode dosimeter was much greater outside of a field relative to the beam central axis. Therefore, they proposed that enough knowledge of the relative electron contribution specific to the measurement position and field size is required for measuring a 6 MV x-ray dose outside a field with a diode. The amount of contamination depends on the field size (2, 24, 34, 36, 38, 42) and the energy of primary photons (26, 31, 32). Also, Narayanasamy et al. declared that ion chamber readings when using 5, 10, and 15 mm bolus were about 31%, 22%, and 10% of the readings for open beam (30) Lye et al. (33) showed that the lead cutouts applied for kilovoltage radiotherapy increase dose due to electron contamination at the edges of the radiation field at shallow depths. Hence, it can lead to erythema and hyperpigmentation at the border of the treated and untreated area of the patient skin. They suggested wrapping a plastic film around the lead cutout or place under it to remove the electron contamination. They concluded that the increment of edge dose at a particular depth in water is highly energy-dependent (33). Also, according to Bahreyni Toossi et al. (35) findings, the presence of prosthesis or metal devices as spinal fixation rods, dental restoration, and fixed prosthodontics in the patient’s body in radiotherapy with high-energy photons increases the possibility of generating electron and neutron contamination. Therefore, they concluded that the prosthesis should not be exposed to the primary radiation in the treatment planning of high-energy photon therapy of patients with such prosthesis.
To reduce electron contamination in photon therapy researchers have evaluated some methods. For example, Kumar et al. (28) stated that the electron contamination for 60Co photons originates mainly from source shielding and can be decreased by an increase in the distance between source and the dosimeter. Also, Li and Rogers (44) used a 0.1 cm lead filter to remove the effect of electron contamination on percent depth dose for photon fields. They stated that the number of contaminating electrons depends on the distance between the added lead filter and the phantom surface. Accordingly, electron contamination is more considerable at a distance of 30 cm between the foil and the phantom surface compared with a 50 cm distance. Thus, they proposed that the filtering foils should be placed at least 50 cm away from the phantom surface to minimize electron contamination contribution in the depth dose of the photon (44).
As we know, electron contamination influences the percentage depth dose measured at 10 cm for a 10 × 10 cm2 photon beam with SSD of 100 cm or %dd (10). In this regard, Chegeni et al. (40) showed 10% reduction of the deliverd dose to the target volume which received 80% of the prescribed dose, in the absence of electron contamination. Also, Li and Rogers (44) concluded that using a 0.1 cm lead filter can reduce the effect of contaminating electrons on surface dose more than 95% for photons with energy ranges from 60Co to 50 MV. They showed that to achieve the best results, the lead filter should be placed immediately below the accelerator head (44). Also, Shukla et al. (41) found that tin, copper, and nickel are effective filters for removing nearly 38% of contaminating electrons. Chegeni et al. (39) reported that lead filter decreased electron contamination by about 3.5%, while light elements, such as aluminum and poly methyl metacrylate (PMMA) had no significant effect on electron contamination reduction.
To correct the maximum dose of photon beams for electron contamination, Rogers (45) conducted a Monte Carlo study. They also considered a 1mm lead foil to remove unknown contaminating electron from the linac head. This filter was located 50 or 30 cm away from the phantom surface. Their calculations showed that about 20% of variations in the filter thickness had a negligible effect on the calculated corrections for a maximum dose of photons in the presence of electron contamination from filter compared with open field photons (45). Also, Li and Rogers (44) obtained a cubic function for relating the values of the water-to-air stopping power ratio of an unfiltered photon field to the values of percent depth dose in the filtered beam. They concluded that the variations of stopping-power ratios in unfiltered beams for the same value of percent depth dose in the filtered beam is about 0.2% for all beams (44). Also, Buston et al. (27) showed that blocking trays applied in radiotherapy make electron contamination, which can be absorbed by attenuating materials, placed over patient’s skin.
Contaminating electrons, which result from the interaction of therapeutic photons with the components in the head of a linear accelerator, can increase dose deposition in the treatment field. Therefore, electron contamination can increase the probability of skin and subcutaneous tissue complications during radiotherapy (5, 36). In this regard, applying a magnetic field can be a feasible approach to turn the contaminating electrons away from the treatment field. Using a magnetic field to sweep contaminating electron away can decrease the skin and subcutaneous dose from high-energy photons in radiation therapy (28, 36, 43, 46). Also, another discussed method to reduce electron contamination in literature is replacing a part of the air column between the radiotherapy system head and patient or phantom surface with a helium gas region to reduce contaminating electrons originated from the air (15, 39, 46). In this regard, Chegeni et al. (39) showed that the presence of helium instead of the air column reduced the surface dose by about 10%.
To investigate the effect of magnetic fields on contaminating electrons in radiation therapy, Damrongkijudom et al. (43) conducted a simulation study. They aimed at evaluating the strength of magnetic fields produced by an improved magnetic deflector (Nd2Fe14B magnets) for decreasing the electron contamination of 6 MV x-ray photons. Their results showed up to 34% reduction in skin and subcutaneous dose when using improved magnetic deflector compare with original values for a 20 × 20 cm2 radiation field of 6 MV photon beam (43). Also, Oborn et al. (46) used GEANT4 software to conduct a simulation study for modeling electron contamination and to reduce it in a 1 Tesla MRI-Linac system. They investigated the effect of magnetic deflectors and also helium gas on reducing electron contamination and concluded the efficacy of magnetic deflectors along with helium gas to decrease electron contamination (46).
5. Conclusions
This research summarized the studies on the sources of electron contamination as flattening filter and air column between the collimator and patient plane and measuring or calculating contaminating electron’s contribution to the surface and total dose of radiotherapy. Also, factors affecting electron contamination, such as beam energy, field size, and SSD, were addressed. Furthermore, some methods were studied to remove the electron contamination, including the use of a lead filter and magnetic field, which all have limitations. The lead filter causes the photon beam to harden. Due to high costs, heavy magnets are not clinically effective.
It can be concluded that the amount of electron contamination depends on factors, such as radiation field size, beam energy, and materials placed in the photon path (43). For larger field sizes, the primary beam interacts with more surface area of the collimator and air medium between collimator to the patient plane, which generates secondary electrons and contributes to the surface dose (37). The increased surface dose due to contaminating electrons can introduce damage to the skin and subcutaneous tissue. The excessive delivered doses can cause skin damages, such as erythema, desquamation, and telangiectasia inside or outside the treatment field (5, 43). According to the results of reviewed studies, we can conclude that using the materials with lower atomic numbers and high densities and helium bag simultaneously is an approach for decreasing electron contamination originated from the head of accelerator and secondary collimators.