1. Background
Systemic lupus erythematosus (SLE) is an autoimmune disorder of unknown etiology that affects several organs and causes tissue damage by producing and depositing autoantibodies and pathogens immune complexes in tissues and cells (1). The immune system’s natural and adaptive components are both involved, with the latter including both B and T cells. Sun exposure, medicines, chemical compounds, and hormones are all important environmental factors that have been reported to exacerbate the disease (2). Moreover, an increased incidence of LE in siblings confirms a genetic predisposition (3).
Currently, SLE is one of the most common diseases of the immune complex in developed countries, and its prevalence in the United States is 7.22 per 100,000 population yearly (4). The prevalence of lupus in Iran is estimated at 40 per 100,000 according to a large population-based study of the Rheumatic Diseases Control Society; that is, one in 2,500 Iranians is afflicted with lupus (5). Although lupus can occur at any age and affects both sexes, the ratio of women to men is 9:1, with the highest incidence in women of childbearing age (6). It is classified as systemic lupus erythematosus and cutaneous lupus erythematosus (CLE), depending on the anatomical location and course of the disease (2).
Both clinically and histopathologically, oral lesions seen in SLE and discoid lupus erythematosus (DLE) have similar characteristics. White striae with a radiating direction are typical clinical lesions, and these may sharply terminate at the center of the lesions, which has a more erythematous presentation. The gingiva, buccal mucosa, tongue, and palate are the most commonly affected locations. Erythematous lesions can occur in the palatal mucosa, and white structures may not be seen. Lupus patients have oral lesions that look similar to lichen planus; therefore, marginal gingival redness and white reticular lesions might be the signs of the disease (7).
Oral mucosa lesions that are associated with LE might be the disease’s first symptom. Oral lesions have been recorded in around 20% of people with LE, while the percentages range from 9% to 45%. Oral mucosal lesions are often a reflection of disease activity. The symptoms of this disease vary from mild to severe. A unique characteristic of SLE is that it is periodic, including recurrent periods with exacerbation of symptoms and remission periods with milder symptoms (6, 8).
The main manifestations of systemic lupus erythematosus disease occur in connective tissue and blood vessels. As known, SLE is diagnosed based on a set of symptoms such as pain, and signs such as fever, blood tests, and urine tests after ruling out other diseases. The American Rheumatological Association has published a set of 11 diagnostic criteria for SLE that can be used to differentiate it from other diseases (2, 9). For a definitive diagnosis of SLE, the patient must fulfill at least four of these 11 criteria at each time of the disease (2, 9) (Box 1).
| American College of Rheumatology Criteria for Systemic Lupus Erythematosus |
|---|
| 1. Malar rash |
| 2. Discoid lesions |
| 3. Photosensitivity |
| 4. Presence of oral ulcers |
| 5. Non-erosive arthritis of two joints or more |
| 6. Serositis |
| 7. Renal disorder |
| 8. Neurologic disorder (seizures or psychosis) |
| 9. Hematologic disorder (hemolytic anemia, leukopenia, lymphopenia, or thrombocytopenia) |
| 10. Immunologic disorder (anti-DNA, anti-SM, or antiphospholipid antibodies) |
American College of Rheumatology Criteria for Systemic Lupus Erythematosus
The criteria are as follows:
Skin Malar Rash: A red skin rash on the cheeks and bridge of the nose (10).
Increased Sensitivity to Sunlight (Photosensitivity): An increase in the skin’s response to sunlight (11).
Lupus Discoid: A round, coin-shaped rash with a raised, scaly surface that appears on the face, scalp, ears, and chest or arms. The lesions remain after healing (12).
Mucosal Ulcers: These are small sores that form in the mouth or nose. They are usually painless, but nasal ulcers may lead to nosebleeds (13, 14).
Arthritis (Inflammation of Joints): Arthritis causes pain and swelling in the joints of the hands, wrists, elbows, knees, and other joints of the hands and feet. The pain may be migratory; that is, it may involve two similar joints on either side of the body. Arthritis in lupus does not cause permanent changes and deformities (15).
Pleurisy (Inflammation of the Pleura): Pleurisy refers to the inflammation of the pleura, which surrounds the lungs, and pericarditis refers to the inflammation of the pericardium, which surrounds the heart (16).
Kidney Involvement: It occurs in almost all children with lupus, and can range from very mild to very severe. They are usually asymptomatic at the onset of the disease and only detected on urine and blood tests (17).
Central Nervous System (CNS): The involvement of CNS includes headaches, seizures, and neuropsychiatric manifestations such as impaired concentration and memory, mood disorders, depression, and psychosis (18).
Blood Cell Disorders: These disorders are caused by autoimmune antibodies (autoantibodies) that attack blood cells (19).
Immunological Disorders: These disorders are caused by the presence of circulating autoantibodies (autoantibodies such as ANA, Anti-DNA, Anti-Sm) present in lupus (20). However, a positive ANA test alone does not prove lupus, as it may test positive for other diseases and even be seen as weakly positive in 5% - 15% of healthy children (21).
Lupus can cause damage to the inside of the mouth, which could be caused by the disease itself or by the side effects of the medications used to treat it. Approximately, 40% of lupus patients experience oral symptoms (22). Besides, LE manifests itself clinically in a variety of pictures, which is reflected in histology, as well. The most common histopathologic features of LE are (1) hyperkeratosis with keratotic plugs; (2) rete process atrophy; (3) significant inflammatory infiltration; (4) lamina propria edema; and (5) thick patchy or continuous PAS-positive juxta-epithelial deposits (23, 24).
These sores are not easily distinguished from other common oral diseases, such as aphthous ulcers, although they occur with increasing incidence in the palate and oropharynx and are painless. These lesions may be difficult to distinguish from other common mucosal disorders such as oral candidiasis or lichen planus, especially if there are few lesions and no systemic or cutaneous involvement (1, 25).
The oral lesions may respond to the disease’s systematic therapy, but they must first be assessed. Topical steroids, such as clobetasol propionate gel 0.05%, betamethasone dipropionate 0.05%, or fluticasone propionate spray 50 g aqueous solution, should be recommended when symptomatic intraoral lesions are present (12). Additionally, immunosuppressive medications are utilized as an adjuvant to minimize the dosage of corticosteroids (12).
2. Objectives
One of the lupus symptoms would be oral manifestations. As a result, we decided to undertake to determine the prevalence of oral signs in lupus patients referred to Shahid Mohammadi Hospital in Bandar Abbas.
3. Methods
In this descriptive cross-sectional study, we examined patients with lupus who were referred to Shahid Mohammadi Hospital in Bandar Abbas from 2018 to 2019. Patients referred to the center had oral manifestations and were thoroughly examined by a dentist. The inclusion criteria for patients in our study were a definitive diagnosis of lupus by a physician (according to the American College of Rheumatology criteria for the classification of lupus) and having at least one oral manifestation of the disease. The exclusion criterion included the presence of an underlying disease other than lupus (6).
A dentist examined eligible individuals after excluding those who were not qualified or did not want to attend the study. The patients were first asked to provide information such as the age of onset, age of diagnosis, and date of the oral lesion. The dentist then examined the patients on the dental unit using a disposable dental mirror and catheter and provided information about the location of the lesion (buccal-lip-hard palate-dorsal of the tongue) and manifestations of the lesion (ulcer, red skin lesions, white and red skin lesions, non-cleansing white lesions), and then recorded them in a pre-prepared checklist. Finally, the data were entered into SPSS software version 22 and analyzed by chi-square and ANOVA tests. This dissertation has the ethics code of IR.HUMS.REC.1398.364.
4. Results
The data obtained were put into SPSS version 22 software and analyzed by the chi-square and ANOVA tests. After collecting data and identifying patients with exclusion criteria, a total of 76 patients were evaluated. Of these, 23 (30.2%) were males, and 53 (69.8%) were females. The participants’ mean age was 42.9 ± 6.1 years, including 34 over 45-years-old, 27 between 35 and 45-years-old, and 15 under 35-years-old. The mean duration of lupus onset in patients studied in this research was 5.05 ± 0.6 years. Besides, 44 patients were diagnosed between one and five years ago, and 32 patients were identified more than five years ago. The mean frequency of oral lesions in participants in this research was 3.6 ± 2.1days, according to the results. A total of 23 individuals reported it within the first five days after the lesion, 43 patients between five and 10 days, and 10 patients after 10 days.
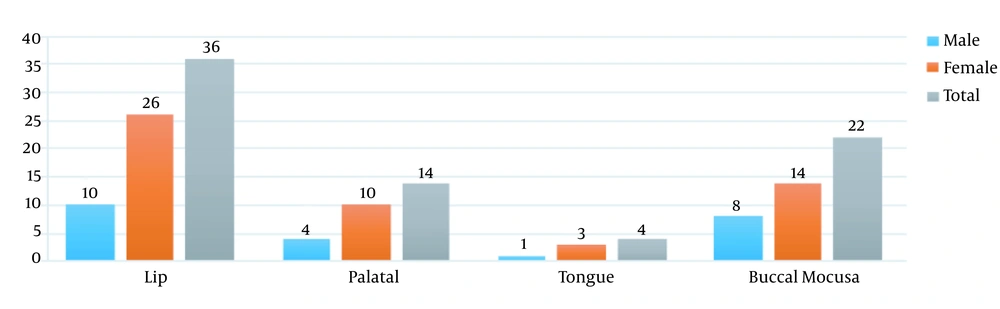
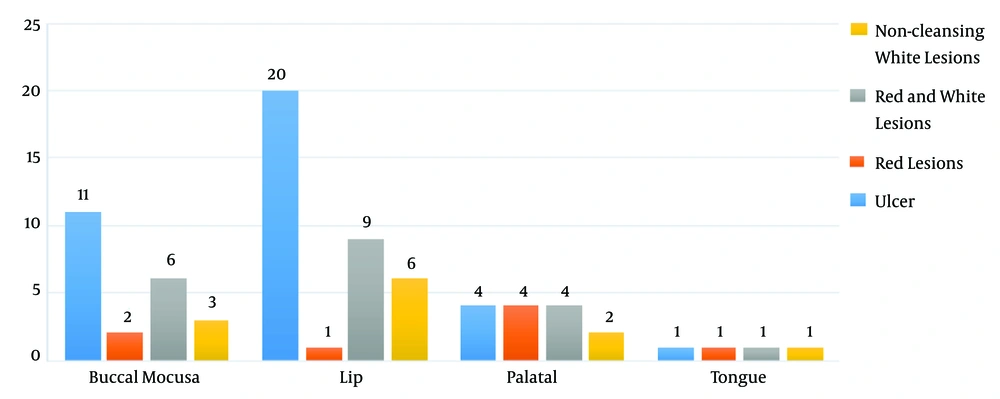
The findings of this investigation concerning the location of oral lesions in patients revealed that the lip was the most common site of the lesion. In addition, all of the patients had active lupus. In 36 patients with lip lesions, 22 patients reported lesions in the buccal mucosa, 14 patients had lesions in the palatal mucosa, and four patients had lesions on the tongue’s dorsal surface (Figure 1). The majority of clinical manifestations in the participants were ulcers, according to the results of clinical manifestations. Besides, 36 individuals had ulcers, 20 had white and red lesions, 12 had white keratotic lesions, and eight had red lesions, according to the examinations (Figure 2).
In evaluating and comparing the locations of lesions in men and women in all areas, we observed a significant difference in the frequency of lesions between men and women, with women having a higher prevalence of lesions than males. The lip area had a P-value of 0.005, the buccal mucosa had a P-value of 0.049, the palatal (hard palate) had a P-value of 0.032, and the dorsal surface of the tongue had a P-value of 0.020, indicating the significance of the differences between men and women in all areas.
In comparing lesions between different age groups in the palatal and tongue areas, we observed a significant difference in the incidence of lesions between different age groups, so that the prevalence of lesions in the group above 45 years was more than that in the other groups. Only in the tongue and palatal areas, we observed a P < 0.05, and a significant relationship was reported (Figure 1).
In comparing clinical manifestations between different age groups of the ulcer group (P = 0.025) and keratotic white lesions (P = 0.032), we found a significant difference in clinical manifestations between different age groups, as the prevalence of lesions in the group over 45 years was higher than that in other groups (Table 1).
| Clinical Manifestations | Age | Cases (%) | P-Value |
|---|---|---|---|
| Ulcer | < 35 | 7 (19.44) | 0.025 |
| 35 to 45 | 11 (30.55) | ||
| > 45 | 18 (50) | ||
| Red lesions | < 35 | 2 (25) | 0.061 |
| 35 to 45 | 2 (25) | ||
| > 45 | 4 (50) | ||
| Red and withe lesions | < 35 | 4 (20) | 0.92 |
| 35 to 45 | 7 (35) | ||
| > 45 | 9 (45) | ||
| Keratotic white lesions | < 35 | 1 (8.33) | 0.032 |
| 35 to 45 | 4 (33.33) | ||
| > 45 | 7 (58.33) |
Relationship Between Clinical Manifestations and Age Groups
Comparing the sites of the lesion with the clinical manifestations, in the buccal mucosal (P = 0.040) and lip (P = 0.003) locations, we found a significant difference between the location of the lesion and clinical manifestations. In both of these sites, the clinical manifestations of the wound were more observed (Figure 2).
5. Discussion
This study aimed to investigate the prevalence of oral manifestations in lupus patients referred to Shahid Mohammadi Hospital in 2018 - 2019. Seventy-six patients with lupus were studied, of whom 23 (30.2%) were males, and 53 (69.8%) were females. The participants’ mean age was 42.9 ± 6.1, which fully confirms that the majority of patients with this disease are women, and all patients referred to Shahid Mohammadi Hospital at that time were active lupus patients. The mean time of onset in the patients studied in this study was 5.05 ± 0.6, and the average time of oral lesion occurrence in the patients was 6.3 ± 1.2 days. The most common site of lesion in patients was their lips, and the most clinical manifestations were in the form of ulcers, which accounted for 45% of the patients.
Comparing lesion location between men and women, we saw a significant difference between men and women in the lip area (P = 0.005), buccal mucosa (P = 0.049), palatal (P = 0.032), and tongue (P = 0.020), and in clinical manifestations of the lesion, there was a significant difference between men and women only in the ulcers (P = 0.011) and keratotic white lesions (P = 0.038).
Comparing lesion sites between different age groups, only in the tongue and palatal areas (hard palate), we observed a significant relationship so that the prevalence of lesions in the group older than 45 years was higher than that in the other groups. Comparing clinical manifestations between different age groups of the ulcer group (P = 0.025) and keratotic white lesions (P = 0.032), we observed a significant difference in clinical manifestations between different age groups. In examining and comparing the site of lesions by different groups of clinical manifestations in the buccal (P = 0.040) and lip (P = 0.003) mucosal areas, we observed a significant difference in the prevalence of lesions by clinical manifestations; in both areas, lesions were observed most frequently.
In a study conducted by Hammoudeh et al. (26), they examined 77 patients referred to the rheumatology ward of a Qatari Hospital and found that oral lupus manifestations were 2.4% - 88.1% for soft palate ulcers, angular cheilitis, and oral candidiasis. They reported the rate of ulcers more than that of other manifestations, which was consistent with the results of our study, in which ulcers were the most common clinical finding in the mouths of patients whose lupus erythematosus was systemic, followed by white, red, and keratotic white lesions, in sequence (26).
Zakeri et al. (27), by examining 70 patients with systemic lupus erythematosus (63 females and seven males) in the age range of 15 - 70 years, concluded that 61.4% had oral lesions, and the most common findings were red lesions (35.08%), white lesions (21.05%), pigmentation (19.29%), ulcers (52.5%), angular cheilitis (10.52%), and white and red lesions (3.54%). These results are entirely consistent with the results of our study. Wounds are the most common clinical manifestations both in our study and that of Zakeri et al. (27). However, Zakeri et al. (27) did not find any significant relationship between oral manifestations and sex, age, or duration of disease, which is not consistent with our study because in our study we observed significant relationships with both sex and age groups. For example, in our study, we had a significant difference between males and females in the development of lesions in the lip area (P = 0.005), and concerning lesion areas, there was a difference between different age groups in the tongue palatal areas. These differences can be attributed to differences in the study population and that the study of oral lesions in the study of Zakeri et al. (27) was one of the sub-objectives of their study.
In another study by Lopez-Labady et al. (28), of 90 patients, 10 showed disease-related oral lesions. Sixteen lesions were examined. We observed oral ulcers with white bands in five patients, erythema in five patients, and homogeneous white plaques in one patient. These results were consistent with our study results in which ulcers were more common than other oral manifestations, and they can be perceived as the most common clinical manifestations of lupus in the mouth (28)
A study by Urman et al. (29) stated that oral mucosal ulcers occurred in 26% of patients, which was the most common lesion in the oral area. Oral ulcers were associated with increased overall clinical severity, although this was not associated with significant changes in the levels of C3 titers, anti-DNA antibodies, and anti-nuclear antibodies. The study results by Urman et al. (29) were consistent with the results of our study.
In general, the studies reviewed were descriptive, and the difference between our study and these studies was that in addition to being descriptive, our study also analyzed the data, but a few studies have analyzed this issue. Epidemiologists can use this study as a reference for this issue.
5.1. Conclusions
There are several reports from different parts of the world regarding the prevalence and characteristics of lupus. Oral symptoms are usually the first signs of the disease. Accordingly, dentists play an important role in diagnosing autoimmune pathologies. In fact, early detection can have a decisive role in improving the quality of treatment strategies and quality of life. Thus, dentists need to increase their diagnostic ability in this regard, and since the most clinical manifestations of the wound and the most involved area of the lips, these two points should be considered more than others.