1. Background
Campylobacter species are one of the four key global causes of foodborne and diarrheal diseases. The natural reservoirs for these organisms are warm-blooded animals such as poultry and sheep. Contaminated milk, water, and avian carcasses with campylobacter are generally known as the main transmission route of this bacterial species to human society. This contamination can occur during slaughtering process, infection with polluted feces, and poultry farm procedures. However, according to different studies, consumption of undercooked contaminated poultry is a major contributor for campylobacteriosis in humans (1-6). According to numerous reports, the occurrence of campylobacteriosis has increased in the world, especially in the Middle East regions and Asia (6, 7). As per the worldwide record, 20% - 35% of human diarrheas are caused by campylobacter species. In Iran, the prevalence rates of Campylobacter jejuni (C. jejuni) and Campylobacter coli (C. coli) in animal sources (34.71%, 68.73%), food products (42.18%, 72%), and human clinical specimens (7.77%, 25.84%) were high (4). In fact, campylobacteriosis is a zoonosis disease caused by Campylobacter species with common clinical symptoms and different complications. Antibiotics are considered as important and traditional agents for therapeutic or prophylactic purposes to control campylobacter infections in aviculture or poultry farms. Unfortunately, discontinuing administration of the offending antibiotic for treatment has led to appearance of antibiotic-resistant strains (4, 7-10). Today, an alternative approach to control and treat campylobacter infections is the usage of anti-campylobacter bacteriocins, campylobacter vaccines, and probiotics as food supplements in the poultry farms (11-22). Some studies showed the positive effects of probiotics on animals’ immune systems (23-29). In addition, research on the epidemiology of infectious diseases, especially zoonosis microorganisms, is essential for designing a suitable plan, effective control measures, and treatment strategies in poultry farms and slaughterhouses to reduce the prevalence of campylobacteriosis in human society.
2. Objectives
Although there are many epidemiological studies performed in Iran on Campylobacter species, information about the prevalence rate in poultry carcasses in Fars province is limited. Accordingly, this research aimed to evaluate the frequency of C. jejuni and C. coli among poultry carcasses through phenotypic and molecular methods.
3. Methods
3.1. Sample Collection
In this cross-sectional study, 370 samples of poultry carcasses were collected according to sample size formula (n = Z2P (1-P)/d2) from five slaughterhouses in south of Iran from January 2019 to June 2019. Twenty g of each sample was collected in a sterile vial, then transferred and kept at 4°C in Zoonosis Research Center of Jahrom University of Medical Sciences (JUMS) for next and molecular experiments.
3.2. Inclusion and Exclusion Criteria
Inclusion criteria were poultry carcasses directly provided after slaughtering process, and exclusion criteria were poultry products in the poultry farms, stores, and other avian carcasses.
3.3. Microbiological Assays
Sample collection and microbiological assays were determined according to Henao et al. (30) with some modification. Each sample was washed with 0.1% sterilized peptone water and centrifuged at 10000 × g for 20 minutes. The sediment was cultured in the Exeter broth (polymyxin 2500 IU/L, rifampin 5 mg/L, amphotericin B 2 mg/L, trimethoprim 10 mg/L, and cefoperazone 1.5 mg/L) and incubated at 42°C for 48 hours under microaerophilic conditions. Then, 100 µL of each sample was loaded on a selective Skirrow Agar (contained defibrinated horse blood 5%, polymyxin B 250 mg/L, vancomycin 10 mg/Lit, and trimethoprim 5 mg/L). After incubation for 48 hours, campylobacter colonies were identified with bacteriological methods such as colony features, gram staining, oxidase tests, nitrate reduction, catalase test, and hippurate hydrolysis test. In this study, C. coli (RTCC 2541) and C. jejuni (ATCC33560) strains were included as positive controls for both phenotypic and molecular identification. In addition, we provided the strains from Razi Vaccine and Serum Research Institute (Tehran, Iran) and Mast International Co. (USA). We selected the media plates with suspected colonies for polymerase chain reaction (PCR) assays.
3.4. DNA Extraction and Primers Information
In this study, four primer pairs were selected from the relevant articles and checked at https://blast.ncbi.nlm.nih.gov/Blast.cgi as follows: a 16s rRNA for detection of Campylobacter spp. (31), an asp (aspartokinase gene) for detection of C. coli, a hipo (hippuricase gene) for detection of C. jejuni (32), and a 16s Universal primer for internal control (15). For molecular assay, the primers and DNA extraction kit were purchased from Cinna Gen Inc., Tehran, Iran, and Iranian Nedaye Fan Company (Cat no.: PR881613), respectively.
3.5. Molecular Assays
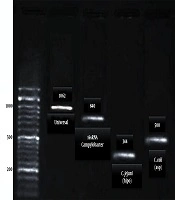
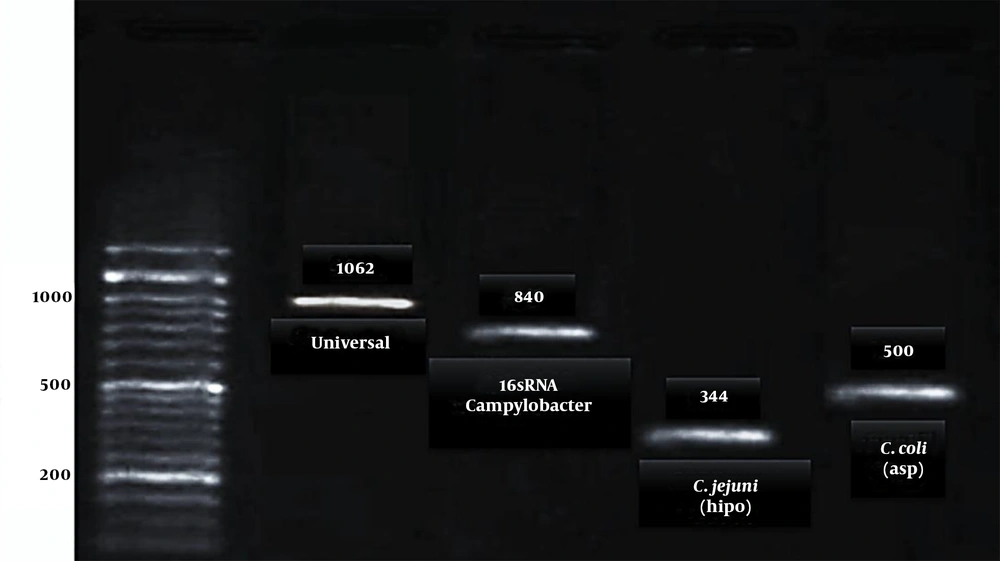
The PCR was carried out using Jenet Bio kits (Cat no.: G-2000). Table 1 shows the information about primer sequences, annealing temperature, and amplicons size. The PCR micro tube contain; buffer (2.5 µL), Template (2 µL), Taq polymerase (1.25 units), MgCl2 (1.5 mM), mixed dNTP 10 mM (1 µL), and primers (1 µL of 10 picomoles of each other and distilled water) and sterilized distilled water to complete the reaction volume (25 µL). The results were detected by gel electrophoresis and gel documentation (33).
| Target Gene | Primer Sequence | Annealing Temperature | Amplicon Size, bp | Reference |
|---|---|---|---|---|
| Universal | F: 5’- GGA GGC AGC AGT AGG GAA TA-3’ | 52°C | 1062 | (34) |
| R: 5’ TGA CGG GCG GTG AGTACA AG-3’ | ||||
| Campylobacter spp. (16srRNA) | F:5’-GGAGGATGACACTTTTCGGAGCG-3’ | 52°C | 840 | (31) |
| R: 5’-TCGCGGTATTGCGTCTCATTGTATATGC-3’ | ||||
| C. jejuni (hipo) | F: 5’- GAC TTC GTG CAG ATA TGG ATG CTT-3’ | 52°C | 344 | (32) |
| R: 5’ GCT ATA ACT ATC CGA AGA AGC CAT CA-3’ | ||||
| C. coli (asp) | F: 5’- GGT ATG ATT TCT ACA AAG CGA G-3’ | 52°C | 500 | (32) |
| R: 5’ ATA AAA GAC TAT CGT CGC GTG-3’ |
Characterization of Primers (F: Forward Primer, R: Revers Primer)
3.6. Data Analysis and Statistics
Statistical analyses, calculated by chi-square test and (P < 0.05) using SPSS 21 software.
4. Results
According to biochemical and microbiological analyses, out of 370 poultry carcasses, 167 (45%) samples were not contaminated, and 203 samples were recognized as a Campylobacter species. Based on the molecular examination of these samples with suspected colonies in bacteriological examinations, 73 (19.7%; C. coli) and 130 (35.1%; C. jejuni) species were recognized as Campylobacter spp. (Figure 1).
5. Discussion
Several studies reported that the prevalence and antibiotic-resistance rates of C. coli and C. jejuni are on the rise in Iran (4). According to our results, molecular detection confirmed the prevalence of these bacterial species among poultry carcasses in south of Iran. Today, Campylobacter spp. have a broad spread among aviculture and poultry farms in different countries. However, poultry carcasses and different avian products might be contaminated through animal feces during slaughtering technics in slaughterhouses. Also, these zoonotic bacteria are transmitted to the food chain and can be spread among human society. In the invasive form, Campylobacter can attack the intestinal mucosa cells and damage the tissues or remain without symptoms and keep shedding through carrier people. Generally, in this condition, using antibiotics is necessary to eliminate the carrier state and effective treatment. In addition, novel anti-Campylobacter treatments are suggested to decrease colonization in avian products and reduce campylobacteriosis in humans society. Today, different procedures are suggested to control the bacterial population in the poultry farms, including the use of probiotics to reduce the colonization in avians and poultries as a feed additives or supplements besides decreasing the incidence of antibiotic-resistant strains and making poultry safer for human consumption (17, 28, 29, 35-38). In Iran, some poultry farms used these supplements in the food chain of young poultries with sterile gastrointestinal tract at birth time (28). Since information about epidemiology and frequency of the Campylobacter species among poultry carcasses after slaughtering process in our region is very limited, in this molecular study, we selected five slaughterhouses that process and sent the poultry products to different regions of the province. According to our results, molecular analysis confirmed the high prevalence of C. coli and C. jejuni among our samples compared to other studies in Iran. For instance; Abdi-Hachesoo et al. (39) showed the high contamination rate of C. jejuni (43/83) and C. coli (40/83) that isolated from poultry carcasses and other studies reported the occurrence of Campylobacter spp. in poultry meats in Tehran (Capital of Iran) and Mashhad (Khorasan Province) 63.2% and 76% respectively.). Furthermore, Taremi et al. (40) and Rahimi and Ameri (41)reported the incidence of Campylobacter spp. as 45.5% and 43.5% in ShahreKord, respectively (39-43). These findings are parallel with our results and disclosed a high prevalence of this bacterial species in Iran. Also, other studies showed the frequency of this bacterial species from Canada (62.4%), Korea (68.3%), and Japan (40% - 77%) (44, 45). Furthermore, a high incidence of campylobacter spp. in poultries was reported from Grenada, Reunion Island, China, and Spain (13-16). The high occurrence of zoonotic Campylobacter species in different countries indicates that poultry farms and slaughterhouses have different methods for slaughtering processes (17, 18, 39). Our study demonstrated that these products are a significant reservoir for C. jejuni and C. coli and increase the risk of transmission of this bacterial species to human society. Furthermore, this finding suggested that the revision of the poultry food programs, using probiotics as a nutritional supplement to health-promoting effects of poultries, and choosing suitable antibiotics to effective control of these bacterial species among animals are indispensable. in order to achieve the above objectives, it is hoped that further epidemiological studies be conducted to determine the frequency of Campylobacter species in other provinces of Iran.
5.1. Conclusions
The findings of this study indicated that consuming poultry carcasses is a potential public health risk in south of Iran regarding foodborne campylobacteriosis. Moreover, these data may assist in effective prevention of transmission of these bacterial species from slaughterhouses to human society, production of healthy animal food products, and revising treatment guidelines for poultries.