1. Background
Urinary Tract Infection (UTI) is characterized by positive urine culture and is one of the most common bacterial infections in children. During the first year of life, its prevalence is 0.7% in girls and 2.7% in uncircumcised boys, and 10% in children aged 4 - 7 years. Besides, UTI is more common in premature infants than in term infants (1-4). Major UTIs are mainly caused by bacteria, and treatment with intravenous antibiotics is necessary for children with severe systemic symptoms. However, the increasing resistance of uropathogenic bacteria in developed and developing countries is worrying (3, 5). If unrecognized, it leads to complications such as high blood pressure, kidney tissue damage, and chronic renal failure (3, 6). Female gender, uncircumcised male, pregnancy, anatomical abnormality, vesicoureteral reflux, constipation, toilet training, sexual activity, neuropathic bladder, voiding dysfunction, and obstructive uropathy are the risk factors of UTI (3).
Zinc is a trace element that plays a critical role in many cell functions in the body (7). Among the physiological functions of zinc are growth, sexual maturity, cell division, and reproduction of immune system activity so that growth retardation, dermatitis of the extremities and around orifices, impaired immunity, poor wound-healing, hypogonadism, and diarrhea are observed in zinc deficiency (6-9).
In studies, zinc consumption has been effective to reduce the fever period, accelerate recovery in pneumonia in children, and reduce the incidence and severity of diarrhea, pneumonia, and possibly malaria. Also, in developing countries, children with diarrhea may benefit from receiving zinc supplements, especially if they are malnourished (5, 10, 11). In reviewing study sources, very few studies have been performed to investigate the effect of zinc on the incidence of UTI. In some studies, zinc deficiency was known to be effective in the development of UTI. However, in a study that examined the effect of zinc supplement administration on the treatment of UTI, it did not affect the duration of fever and the time of negative urine culture (6).
Due to the importance and high prevalence of UTI in children and the costly long-term consequences, and the risk of developing antibiotic-resistant strains in uropathogenic bacteria, it is clear that the prevention of this infection or its effective treatment can greatly help improve the quality of life and the health of the community. Also, due to the important role of zinc in the immune system and prevention of some infections in children and the scarcity of studies investigating the relationship between serum levels of zinc and the incidence of UTIs in children, we decided to conduct a study comparing the serum zinc level in children with febrile UTI and healthy children. If zinc deficiency is a risk factor for UTI, it is possible that taking zinc supplements in child health care programs has a significant effect on preventing UTI and its complications.
2. Methods
This case-control study was performed in the winter of 2020 in Abuzar hospital of Ahvaz. After obtaining permission from the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, 120 children without a history of previous hospitalization or underlying disease and without malnutrition (based on growth percentiles), who were between the ages of 3 and 120 months, entered the study in two groups after obtaining parental consent. The case group included 60 children, who were admitted due to febrile UTI and had normal urinary tract system ultrasound. The control group included 60 healthy children with no history of underlying disease. The diagnosis of UTI was confirmed by positive urine culture. After enrollment, two intravenous blood samples were taken from each child under sterile conditions. In the laboratory, after centrifugation, the serum was isolated from the sample and transferred to micro-tubes by samplers with acid washed tips and kept at -21 °C temperature until the zinc level was measured. Zinc was measured by an auto-analyzer (BT 300 machine, Italy). If there was dehydration in the physical exam or hemolysis or hyperlipidemia was reported by the laboratory, the child was excluded from the study. In this study, the normal serum levels of zinc were considered 60 - 90 µg/dL for children under one year of age and 80 - 110 µg/dL for children aged one to 10 years (12-14).
2.1. Statistical Analysis
For analysis, the data were entered in SPSS version 22 software, and t test, chi-square, Pearson, Spearman, and ANOVA tests were used. A P value of less than 0.05 was considered significant.
3. Results
A total of 120 children, including 70 girls (58.3%) and 50 boys (41.7%) in two groups (control and case) were included in this study. There was no statistically significant difference between the two groups in terms of age (P = 0.07) and sex (P = 0.19).
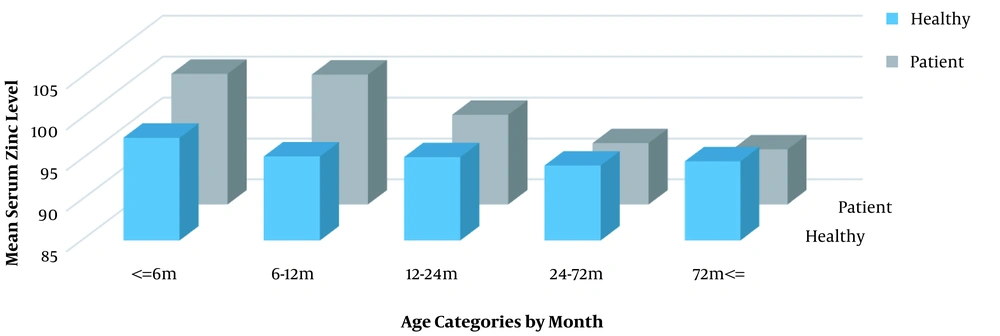
Comparing the serum zinc levels between the two groups, no statistically significant difference was observed in any of the age groups (P1 = 0.777, P2 = 0.359, P3 = 0.887, P4 = 0.576, and P5 = 0.385) (Figure 1). The minimum and maximum serum zinc levels were 70 µg/dL and 146 µg/dL, respectively, and the mean value was 95.05 ± 11.34 µg/dL in the control group and 94.98 ± 12.24 µg/dL in the case group; this difference was not statistically significant (P = 0.98).
The mean serum level of zinc was 96.157 ± 12.875 µg/dL in girls and 93.420 ± 9.856 µg/dL in boys, and this difference was not statistically significant (P = 0.210). Also, the serum zinc levels were not statistically significant between the two sexes in the case group (P = 0.235) and the control group (P = 0.382).
Zinc deficiency was observed only in children over one year of age (1.66% in the control group and 3.33% in the case group), and this difference between the two groups was not statistically significant (P = 0.35). Zinc deficiency was not observed in any of the groups in children under one year of age.
In the case group, the lowest recurrence rate of UTI was one time, and the maximum was three times, and there was no statistically significant relationship between the serum levels of zinc and the frequency of UTIs (Table 1). However, there was a statistically significant relationship between the frequency of UTI and age in both sexes (P = 0.000, r = 0.573), and the rate of UTI increased with increasing age more in boys (P = 0.004, r = 0.782) than in girls (P = 0.000, r = 0.505). There was no statistically significant relationship between the age and the serum level of zinc according to the Pearson correlation coefficient (P = 0.071, r = -0.165).
| Variables | Mean | SD | P Value |
|---|---|---|---|
| Thrombocytosis | 357.58 | 100.80 | 0.726 |
| Leukocytosis | 12630 | 5650 | 0.338 |
| Length of hospital stay | 7.45 | 3.08 | 0.093 |
| Frequency of urinary tract infection | 1.25 | 11.74 | 0.499 |
Relationship Between Serum Zinc Level and Severity of Thrombocytosis, Leukocytosis, Length of Hospital Stay, and Frequency of Urinary Tract Infections
Weight comparison between the case and control groups was done in each age group, and no statistically significant difference was observed in any of the five categories (P1 = 0.373, P2 = 0.279, P3 = 0.471, P4 = 0.196, and P5 = 0.222). The differences in the serum level of zinc by sex between the age groups were assessed; these differences were not statistically significant in any of the five age groups (P1 = 0.581, P2 = 0.274, P3 = 0.887, P4 = 0.569, and P5 = 0.654). In the age groups under 72 months (except for the age group of 6 - 12 months), the incidence of UTI was higher in girls, and this difference was statistically significant (P1 = 0.01, P3 = 0.002, and P4 = 0.001). Also, in the age groups above 72 months and 6 - 12 months, the incidence of UTI was still higher in girls, but it was not statistically significant (P2 = 1 and P5 = 0.055).
In the study of the relationship between the serum levels of zinc and weight in different age groups, it was observed that only in the age group of 24 - 72 months, there was a statistically significant linear relationship between the serum zinc level and child weight, and no statistically significant relationship was observed in other age groups (Table 2). This evaluation was repeated non-parametrically (Spearman), which had similar results.
| Age Groups by Month | P Value | r Value |
|---|---|---|
| ≤ 6 | 0.779 | -0.083 |
| 6 - 12 | 0.996 | 0.001 |
| 12 - 24 | 0.153 | -0.440 |
| 24 - 72 | 0.033 | 0.305 |
| ≥ 72 | 0.377 | 0.174 |
Correlation Between Serum Zinc Level and Weight in Different Age Groups Using Pearson Correlation Coefficient a
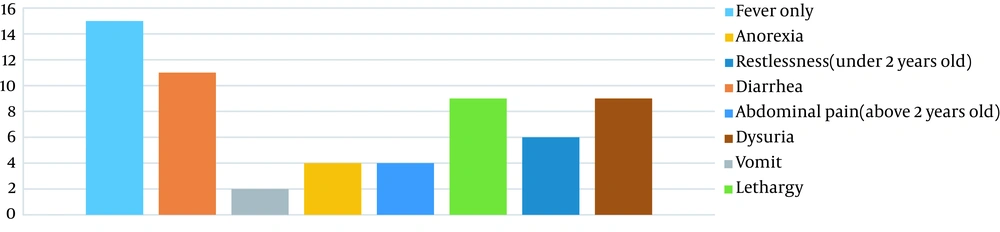
In the group of patients, the frequency of each of the initial symptoms, in addition to fever, was assessed (Figure 2). There was no statistically significant relationship between the serum levels of zinc and the incidence of any of the symptoms (P = 0.271). In the group of patients, 91.1% of them were term (GA > 37 weeks), and 8.9% were preterm (GA < 37 weeks), but the serum zinc level had no statistically significant relationship with gestational age in children under two years old (P = 0.666) or over two years old (P = 0.475).
There was no statistically significant relationship between the serum zinc level and the intensity of increases in ESR (P = 0.803) or CRP (P = 0.531). The relationship between the severity of leukocytosis, thrombocytosis, and the length of hospital stay was investigated, none of which was statistically significant (Table 1).
4. Discussion
This study aimed to compare the serum zinc levels between children with febrile UTI and healthy children. In reviews to find related articles, unfortunately, we had access to few articles, especially those published in the last 10 years. A few studies have examined the association between serum zinc levels and UTI, and very few studies have been performed in children, which proves the importance and value of this study. In this study, it was found that there was no statistically significant difference in the serum zinc levels between the two groups (case and control groups), as well as different age and sex groups. Serum zinc levels were not statistically significantly related to weight in various age groups, except for the age group of 24 - 72 months, which was directly linearly related to weight. Febrile UTI was high in girls in all age groups, but this difference was not statistically significant in the age groups of 6 - 12 months and over 72 months. There was a statistically significant direct linear relationship between the age and the frequency of UTI, which was stronger in boys. But, there was no statistically significant relationship between the zinc level and gestational age, the severity of leukocytosis and thrombocytosis, severity of increases in ESR or CRP, and the duration of hospitalization.
In the study by Zabihi et al., serum zinc levels in children with UTI, regardless of age and sex, were significantly lower than that in the control group (P = 0.001). Because there was a statistically significant difference between the two groups in terms of age (P = 0.035) and sex (P = 0.01) and this difference was evident over the age of one year, multivariate binary logistic regression was used and showed that zinc deficiency increased the risk of UTI in children participating in the study by eight folds (15).
In a study by Noorbakhsh et al., the serum levels of zinc and vitamins A and D were assessed in children aged six months to five years. In children with UTI, serum zinc levels were statistically significantly lower than that in the control group (P = 0.05). However, the levels of vitamins measured between the two groups were not significantly different although they were lower in the patient group (P for vitamin A = 0.40, P for vitamin D = 0.90) (16).
In a study by Farzadmanesh, it was found that the serum levels of zinc (P = 0.001) decreased and copper (P = 0.001) levels increased in children with acute pyelonephritis (17). In a study by Mohsenpour et al., there was a weak association between age and serum zinc levels (P = 0.045, r = -0.205), and serum zinc levels decreased with age. Zinc levels were also lower in patients with recurrent UTI than in controls (P = 0.01). In this study, the mean age was 53.37 ± 19.2 years in the case group and 52.7 ± 19.33 years in the control group. Also, the study population consisted only of females who were studied in two groups of 48 people as the case and control groups (4).
While in our study, the age range was 3 - 120 months, and children of both sexes were included in the study. Serum zinc levels were not statistically significantly different between the two groups in any of the five age groups. There was a significant relationship between UTI frequency and age in both sexes, which was stronger in boys; but the repetition of UTI was not associated with serum zinc levels.
Today, the role of zinc in regulating the activity of the immune system has been proven. On the other hand, immunodeficiency has not been clearly identified as a risk factor for UTI (6, 18). Therefore, zinc supplements are now popular for the prevention and treatment of some childhood infections such as acute gastroenteritis (11), and it is possibly a reason for normal zinc levels of our patients, as the previous use of zinc was not an exclusion criterion in our study. Among our limitations in this study are the dissatisfaction of some parents to participate in the study, the limited number of participants in the two groups, and no diet history-taking.
4.1. Conclusion
According to this study, there was no relationship between serum zinc levels and the risk of UTI in children aged 3 to 120 months. Due to the very small number of studies in this field, it is not possible to express a definite opinion about the effect of zinc deficiency on increasing the risk of UTI in children. Due to the importance of the issue, the need for more extensive studies is raised more than before. It should also be noted that due to changes in the lifestyle and consequently changes in the diet of individuals, changes in serum levels of minerals and vitamins during a person’s life are inevitable. Therefore, periodic surveys, with larger samples and in different geographical areas, as well as making comparisons with previous results, can be helpful in many cases, including deciding whether or not to use different types of supplements in health care in different age periods. Due to the importance of zinc in the function of some organs of the body, it is recommended to study the serum level of zinc and the state of zinc deficiency in society.