1. Background
The World Health Organization (WHO) estimates that tuberculosis (TB) is a leading cause of mortality and morbidity worldwide. An incidence of 10.4 million cases and 1.8 million TB-associated deaths were reported in 2015 (1). Bacillus Calmette-Guerin (BCG) was developed by two French investigators, Albert Calmette and Camille Guerin, from 1908 to 1921 by the subculture of Mycobacterium bovis every three weeks for 13 years, remaining the only currently licensed TB vaccine (2). The WHO approved BCG as a vaccine against TB in 1974 (3). The WHO reports that as many as 100 million children are given BCG vaccination each year (4). The BCG vaccine reduces the risk of pulmonary TB up to about 60% and has a significant effect of 80% in preventing meningitis and disseminated TB infection (5).
However, adverse events after BCG vaccination have been described since its introduction, which can be classified as localized or disseminated manifestations (3, 6). Adverse reactions are reported in up to 10% of vaccinees (7). Some local adverse reactions are reported following BCG vaccination, such as local ulceration, inoculation site abscess, keloid reaction, and regional lymphadenitis. Rarely systemic reactions such as osteomyelitis/osteitis and disseminated BCG infection are reported in immunodeficient children (5, 8, 9).
Simple BCG lymphadenitis will regress spontaneously over a few weeks without the need for any treatment. The suppurative form of BCG lymphadenitis can develop into spontaneous rupture and sinus formation, later healed by cicatrization (10). Needle aspiration is helpful in most cases to prevent these complications and hasten the healing process. Surgical excision is recommended only in case of draining sinus or failed needle aspiration due to multiloculated abscess (11-13). In Iran, BCG is routinely administered in the neonatal period under the National Immunization Program and inoculated intradermally at the left arm. Mycobacterium bovis [French (Pasteur) strain 1173-P2] with 1 mg/mL concentration is the vaccine type used in Iran (14).
2. Methods
Infants born from March to June 2017 that received a BCG vaccine at birth were enrolled in this prospective descriptive study. For this purpose, 12 health centers of Ahvaz (southwest Iran) were selected randomly. Infants referred to those health centers for routine health care were enrolled in the study and evaluated for BCG-associated complications. At first, a questionnaire including demographic data, gestational age, and BCG inoculation site was filled for each infant. The study cases were followed up for BCG complications at two, four, six, nine, and 12 months old by experienced health workers, and suspected cases were referred to pediatricians for confirmation and further evaluation. After explaining the study details to the children's parents, their written consent was taken. The Ethics Committee of Ahvaz Jundishapur University of Medical Sciences approved the study (code AJUMS.REC.1392.45). We used SSPS version 13.0 software for data analysis. The chi-square test was used for testing the statistical significance. A p value of less than 0.05 was considered statistically significant.
3. Results
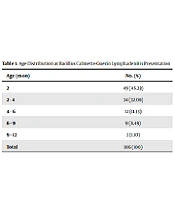
The study enrolled 1506 infants (794 males and 712 females). The BCG vaccine was inoculated in the left arm in 1,091 (72.4%) infants and the right arm in 398 (26.4%) infants. Despite BCG vaccination, 17 (1.13%) infants showed no BCG scar. The BCG vaccine was inoculated in all infants in the first 27 days of birth (87% in the first three days). Four infants presented with significant injection site reactions. Totally, 106 (7.03%) lymphadenitis cases were detected, consisting of 66 (62%) males and 40 (38%) females. The lymphadenitis rate was significantly higher in males than in females (P = 0.024). The mean age at lymphadenitis presentation was 4.28 ± 0.79 months (Table 1). In 93 (87.7%) cases, lymphadenitis involved axillary nodes at the same site of vaccine inoculation. The other involvement sites included the supraclavicular lymph nodes in six (5.67%) cases and both the axillary and supraclavicular lymph nodes in seven (6.6%) cases.
| Age (mo) | No. (%) |
|---|---|
| 2 | 49 (46.23) |
| 2 - 4 | 34 (32.08) |
| 4 - 6 | 12 (11.33) |
| 6 - 9 | 9 (8.49) |
| 9 - 12 | 2 (1.87) |
| Total | 106 (100) |
Age Distribution at Bacillus Calmette-Guerin Lymphadenitis Presentation
Fifty-three (50%) cases developed nonsuppurative lymphadenitis. Of them, 17 (16%) had complete resolution and 25 (23.5%) had partial resolution. In 11 (10.3%) cases, the lymph node size remained unchanged at 12 months of follow-up. Fifty-three (50%) cases developed suppurative lymphadenitis. Of them, 46 (43.4%) cases developed spontaneous drainage, and seven (6.6%) cases underwent needle aspiration (Table 2). Eighty-one cases of BCG inoculation in the left arm and 25 cases of BCG inoculation in the right arm developed lymphadenitis. There was no statistically significant difference between the site of BCG inoculation and the rate of lymphadenitis (P = 0.52).
| Course | No. (%) |
|---|---|
| Complete resolution | 17(16.04) |
| Partial resolution | 25 (23.58) |
| Unchanged | 11 (10.38) |
| Spontaneous drainage | 46 (43.40) |
| Needle aspiration | 7 (6.6) |
Courses of Bacillus Calmette-Guerin Lymphadenitis in the 12-Month Follow-Up
In 202 infants, the gestational age was less than 37 weeks. Of them, 13 cases developed lymphadenitis. Lymphadenitis was observed in 93 cases with more than 37 weeks gestational age. The BCG lymphadenitis rate was not statistically significant between infants with different gestational ages (P = 0.83). After one year of follow-up, no other complications such as osteomyelitis or disseminated BCG infection were observed in our cases.
4. Discussion
Bacillus Calmette-Guerin vaccination is part of the routine immunization program in Iran and other developing countries, with an annual tuberculosis incidence of more than 1%. After injection, the BCG strain starts multiplying at the inoculation site and transfers to the regional lymph nodes via the lymphatic vessels. The vaccination site and the glandular response form a primary complex similar to natural tuberculosis infection (15). Some adverse reactions can occur following BCG vaccination, such as local ulceration, lymphadenitis, osteitis/osteomyelitis, and disseminated BCG disease (16). In our study, 17 (1.13%) cases showed no BCG scar, despite documented BCG vaccination. In a study, 5.6% of the cases had not no scar after BCG vaccination (17). The absence of a scar does not signify the lack of an effective immune response. In our study, lymphadenitis was prevalent in 7.03% of the cases. This proportion is relatively similar to a study from Iran as 5.6% (15) but higher than other reports from Iran 0.93% (18), France 0.1% (17), Latvia 0.1% (7), South Africa 0.5% (19), and Jamaica 1.9% (20).
The lymphadenitis incidence can be affected by several factors, including the virulence of BCG substrain, the viability of the final product, vaccine dose, age at vaccination, and administration technique (3, 4, 16, 19-23). In our study, the relatively high incidence of lymphadenitis can be due to the vaccine strain (Pasteur strain) (14, 24), age at vaccination (newborn infants), and improper administration techniques.
In the present study, 62% of BCG lymphadenitis cases were males. This finding is similar to some reports in which the lymphadenitis prevalence was higher in males than in females (18, 25, 26). However, some studies reported that the lymphadenitis prevalence was the same between males and females (15, 16). On average, in this study, lymphadenitis was presented at 4.28 months of age. In most studies, lymphadenitis developed within 2 - 6 months after birth (2, 15, 16, 23, 26-28). In our study, the commonest involved lymph node was the ipsilateral axillary node, followed by the supraclavicular lymph node. The regional distribution of lymphadenitis was similar to the reports of other studies (3, 7, 12, 15, 16, 23, 28). In our study, the suppurative and nonsuppurative lymphadenopathy rates were equal, similar to studies from the UK (5) and India (6). Other studies showed a higher rate of suppurative lymphadenitis (7, 11, 14, 23, 26). In our study, of 53 cases with nonsuppurative lymphadenitis, 17 resolved utterly, 25 regressed, and 11 remained unchanged after one year of follow-up. Of 53 cases with suppurative lymphadenitis, 46 ruptured spontaneously, and we approached needle aspiration in seven cases of a suppurative lymphadenitis. However, no surgical incision was needed. In our study, there was no statistically significant difference between the BCG inoculation sites, and the rate of lymphadenitis, but one study from Iran reported a higher rate of BCG lymphadenitis following intradermal injection in the right arm compared to the left arm (15).
This study has some limitations. First, we could not detect rare complications like osteomyelitis or disseminated infection because of only one year of follow-up and sample size. Second, we missed some cases with unchanged nonsuppurative lymphadenitis after one year of follow-up.
4.1. Conclusions
The relatively high incidence of BCG lymphadenitis in our region can be due to the vaccine strain, young vaccinees, and improper administration techniques. At least 50% of lymphadenitis cases following BCG vaccination consisted of nonsuppurative lymphadenitis, which is benign and regresses spontaneously without any treatment. However, suppurative lymphadenitis needs treatment. Needle aspiration should be used to prevent spontaneous rupture and sinus formation. Surgical excision is only rarely required.