1. Background
Breast cancer is one of the most common female malignancies worldwide and the principal cause of death among women in developed and developing countries (1).
Early detection and combination treatment such as chemotherapy, radiotherapy, hormone therapy, and surgical procedures can cure breast cancer (2). Surgical procedures, including mastectomy and breast-conserving therapy (lumpectomy), are the most prominent treatment for breast cancer (3). The survival is not different in these two surgical procedures (4), but patients who underwent lumpectomy had a better body image and quality of life (5). Also, a high QOL level could lead to long-term survival (6). Evidence showed that the type of surgery does not affect any part of QOL except sexual function and body image domains (7, 8).
Some women with breast cancer reported physical and psychological distress due to cancer diagnosis and its treatment process that impair the different aspects of cancer patient life, such as family and social life (9). Therefore, patients use various strategies for coping with these stressful conditions. A previous study in Iran showed that coping strategies such as religious beliefs, accepting the reality of the disease, and positive or negative thinking about the disease are essential strategies used by Iranian women with breast cancer. Spirituality is the most common coping strategy (10).
The perceived social support is an essential determinant of patients' capacity with breast cancer to cope with their disease and procedures. It could improve QOL and ease the adjustment to life after treatment (11). The previous study showed that high levels of social support through functional and positive coping reduce depressed and anxious symptoms in patients with breast cancer. Therefore, patients should get social support and be educated on using functional coping strategies (12).
2. Objectives
Researchers believe that the study's results will be helpful for oncology nurses in assessing patients in all aspects and improving their well-being. Therefore, this study aimed to assess the influence of surgical type on QOL, coping strategies, illness adjustment, and social support in breast cancer patients in Zahedan, Southeastern Iran.
3. Methods
In this cross-sectional study, we selected 120 female breast cancer patients using the census method from the Clinical Oncology Department of Khatam-Al-Anbia hospital and the Radiotherapy Department of Ali-Ebne-Abitaleb hospital in Zahedan, Southeastern Iran, from February to August 2020.
These two hospitals handle all cancer patients in Zahedan, and all patients who were referred to these two hospitals and met the inclusion criteria agreed to participate in the study.
The inclusion criteria included a confirmed breast cancer diagnosis, 18 years and older, and willingness to participate in this study. We used a self-administered questionnaire to collect patients' socio-demographic information and clinical characteristics. Also, we investigated the quality of life, social support, coping strategies, and adjustment to illness.
As most of the patients in this study were illiterate or had primary education, we interviewed them privately to fill out the questionnaires.
3.1. Quality of Life
The Quality of Life (QLQ-BR23) consists of 23 questions on four functional scales (body image and sexual functioning, sexual enjoyment, future perspective) and four symptom scales (arm symptoms, breast symptoms, systematic therapy side effects, and being upset by hair loss). We calculated the Item scores of the EORTC QLQ-BR23 according to the EORTC QLQ-C30 scoring manual (13). Each item had a five-point Likert score.
The range of scores for these questions is between 0 and 100. The range of scores for these questions is between 0 and 100. A high score for a functional domain represents a better functioning level, and a high score for a symptom domain represents a worse level of symptoms and more problems. This questionnaire is translated into Persian and validated in the previous study (14).
3.2. Coping Strategies and the Degree of Adjustment
We assessed women's coping strategies with breast cancer and the degree of adjustment to illness using an adjustment to illness measurement inventory for Iranian women with breast can (AIMI-IBC). This instrument had three domains (emotional turmoil, reasonable efforts, and avoidance) with 49 items. Each item had a five-point Likert score, ranging from 1 to 5, and a higher score for each domain reflects frequent use of the coping manner. The cut-off for this scale is 122.5. A mean score of ≥ 122.5 indicates higher adjustment with the illness, and a mean score lower than 122.5 indicates insufficient adjustment. The psychometric property of the AIMI- IBC questionnaire in Iranian women with breast cancer was confirmed (15).
3.3. Multidimensional Scale of Perceived Social Support
Zimet and colleagues developed the multidimensional scale of perceived social support (MSPSS) to measure perceived social support from friends, family, and significant others. This questionnaire consists of 12 items, each with a 5-point Likert-type response format (from 1 = strongly disagree to 5 = strongly agree). The score range is between 12 and 60, and higher scores reflect more perceived social support. The MSPSS had good validity and reliability in a previous study in Iran (16).
3.4. Statistical Analysis
The collected data were analyzed using SPSS software, version 19.0. We used chi-squared analysis to determine the frequency of the type of surgery across the demographic characteristics of patients. We assessed the mean score of quality of life, social support, coping strategy, and illness adjustment among the surgical type variables using the ANOVA and Kruskal-Wallis tests. When one-way ANOVA and Kruskal-Wallis tests were significant, we made multiple comparisons with the least significant difference (LSD).
We performed multivariate linear regression to adjust for covariates to evaluate surgical type's independent effects on QOL, coping strategy, illness adjustment, and social support in the presence of potential confounders. A P-value < 0.05 was considered significant.
4. Results
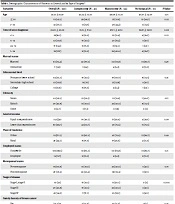
One hundred and twenty patients consented to participate in this study. The mean age of patients was 47.35 ± 10.67 years, and the time since diagnosis was 23.69 ± 20.38 months. Around half of the patients were of Sistani ethnicity (52.8%), followed by Baluch (40.8%) and others (6.7%). Three-fourths of patients were married (75.8), and most were housewives (88.3%). 16.3% of patients had a family history of breast cancer. At the time of the survey, 40.8% of patients had been diagnosed with stage III breast cancer, 34.2% with stage IV, 22.5% with stage II, and 2.5% with stage I. Most (90%) of the patients had received chemotherapy, and 64.2% had received radiotherapy. More than half of the patients underwent mastectomy (53.3%), followed by underwent lumpectomy (34.2%), and 12.5% had not been surgery (Table 1).
| Variables | Overall (N = 120) | Lumpectomy (N = 41) | Mastectomy (N = 64) | No Surgical (N = 15) | P-Value |
|---|---|---|---|---|---|
| Age | 47.35 ±10.67 | 46.32 ± 10.55 | 48.08 ± 10.51 | 47.07 ± 12.09 | 0.71 |
| ≤ 50 | 75 (62.5) | 28 (68.3) | 37 (57.8) | 10 (66.7) | 0.52 |
| > 50 | 45 (37.5) | 13 (31.7) | 27 (42.2) | 5 (33.3) | |
| Time of since diagnosis | 23.69 ± 20.38 | 19.15 ± 17.61 | 27.73 ± 21.72 | 18.87 ± 18.99 | 0.08 |
| < 6 | 22(18.3) | 8 (19.5) | 10 (15.6) | 4 (26.7) | 0.33 |
| 6 - 12 | 43 (35.8) | 17 (41.5) | 20 (31.2) | 6 (40) | |
| 24 - 12 | 17 (14.2) | 8 (19.5) | 8 (12.5) | 1 (6.7) | |
| > 24 | 38 (31.7) | 8 (19.5) | 26 (40.6) | 4 (26.7) | |
| Marital status | |||||
| Married | 113 (94.2) | 40 (97.6) | 60 (93.8) | 13 (86.7) | 0.29 |
| Unmarried | 7 (5.8) | 1 (2.4) | 4 (6.2) | 2 (13.3) | |
| Educational level | |||||
| Primary or lower school | 64 (53.3) | 17 (41.5) | 39 (60.9) | 8 (53.3) | 0.16 |
| Secondary- high school | 43 (35.8) | 16 (39) | 21 (32.8) | 6 (40) | |
| College | 13 (10.8) | 8 (19.5) | 4 (6.2) | 1 (6.7) | |
| Ethnicity | |||||
| Sistan | 63 (52.5) | 23 (56.1) | 33 (51.6) | 7 (46.7) | 0.71 |
| Baluch | 49 (40.8) | 15 (36.6) | 26 (40.6) | 8 (53.3) | |
| Other | 8 (6.7) | 3 (7.3) | 5 (7.8) | 0 (0) | |
| Level of income | |||||
| Equal to expenditures | 33.3))40 | 19 (46.3) | 17 (26.6) | 4 (26.7) | 0.09 |
| Lower than expenditures | 80 (66.7) | 22 (53.7) | 47 (73.4) | 11 (73.3) | |
| Place of residence | |||||
| Urban | 93 (77.5) | 33 (80.5) | 47 (73.4) | 13 (86.7) | 0.46 |
| Rural | 27 (22.5) | 8 (19.5) | 17 (26.6) | 2 (13.3) | |
| Employed status | |||||
| Housewife | 106 (88.3) | 33 (80.5) | 60 (93.8) | 13 (86.7) | 0.11 |
| Employed | 14 (11.7) | 8 (19.5) | 4 (6.2) | 2 (13.3) | |
| Menopausal status | |||||
| Pre menopause | 73 (60.8) | 15 (36.6) | 26 (40.6) | 6 (40) | 0.91 |
| Post menopause | 47 (39.2) | 26 (63.4) | 38 (59.4) | 9 (60) | |
| Stage of disease | |||||
| Stage I, stage II | 30 (25) | 17 (41.5) | 13 (20.3) | 0 (0) | 0.004 |
| Stage III | 49 (40.8) | 17 (41.5) | 26 (40.6) | 6 (40) | |
| Stage IV | 41 (34.2) | 7 (17.1) | 25 (39.1) | 9 (60) | |
| Family history of breast cancer | |||||
| Yes | (16.3) 16 | 6 (14.6) | 10 (15.6) | 0(0) | 0.26 |
| No | (86.7) 104 | 35 (85.4) | 54 (84.4) | 15 (100) | |
| Type of treatment | |||||
| Chemotherapy | |||||
| Yes | 113 (94.2) | 37 (90.2) | 61 (95.3) | 15 (100) | 0.32 |
| No | 7 (5.8) | 4 (9.8) | 3 (4.7) | 0 (0) | |
| Radiotherapy | |||||
| Yes | 77 (64.2) | 31 (75.6) | 40 (62.5) | 6 (40) | 0.04 |
| No | 43 (35.8) | 10 (24.4) | 24 (37.5) | 9 (60) |
Demographic Characteristics of Patients: In General and by Type of Surgery a
Based on the results of the chi-square analysis, patients who underwent lumpectomy more had received more radiotherapy than patients who underwent a mastectomy and were not surgical (χ2 = 6.22, P = 0.04). Most patients who underwent mastectomy had stage III and stage VI of the disease (χ2 = 15.62, P = 0.004).
4.1. Quality of Life
In the functioning scale, the higher mean scores were for body image (78.61 ± 26.69) and future perspective (55.27 ± 26.81), respectively, while the lower mean scores were for sexual enjoyment (14.86 ± 16.84) and sexual functioning (14.16 ± 17.63). On the other hand, on the symptom scale, upset by hair loss (49.16 ± 38.88) and systematic therapy side effects (45 ± 17.42) scored the highest, followed by arm symptoms (30.83 ± 26.73) and breast symptoms (13.63 ± 18.58).
The significant difference across surgical groups was present in the functioning scales (body image, sexual functioning, and sexual enjoyment) and symptom scales (arm symptom). Patients who underwent lumpectomy had the best sexual function, sexual enjoyment, and body image. On the other hand, patients who underwent a mastectomy had severe arm symptoms compared to patients who underwent a lumpectomy. After adjustment for the type of treatment (radiotherapy) and stage of the disease, this finding remained significant.
4.2. Perceived Social Support
The mean score of total social support was 45.71 ± 9.92. Patients reported that they received the highest level of support from their family (18.02 ± 2.76), followed by significant other (14.45 ± 4.27) and friends (13.23 ± 5.21). The statistical analysis showed that perceived social support's (MPSS) mean score and domains had no significant difference among surgical groups (P < 0.05). The finding was uninfluenced even after controlling covariates (Table 2).
| Overall (N = 120) | Lumpectomy (N = 41) | Mastectomy (N = 64) | No Surgical (N = 15) | P-Value | Adjusted P-Value b | |
|---|---|---|---|---|---|---|
| BR23 functional scales c | ||||||
| Body image | 78.61 ± 26.69 | 91.05 ± 16.07 | 67.44 ± 28.97 | 92.22 ± 17.94 | 0.001 d | 0.001 |
| Secual functioning | 14.86 ± 16.84 | 20.32 ± 17.28 | 11.97 ± 16.12 | 12.22 ± 16.01 | 0.03 d | 0.006 |
| Secual enjoyment | 14.16 ± 17.63 | 19.51 ± 18.22 | 11.97 ± 17.17 | 8.88 ± 15.25 | 0.04 d | 0.04 |
| Future perspective | 55.27 ± 26.71 | 60.97 ± 25.71 | 50.52 ± 25.88 | 60 ± 31.37 | 0.11 d | 0.16 |
| BR23 Symptom Scales e | ||||||
| Systemic therapy side effects | 45 ± 17.42 | 47.85 ± 15.64 | 45.16 ± 17.93 | 36.50 ± 18.23 | 0.08 d | 0.18 |
| Breast symptoms | 13.68 ± 18.58 | 18.29 ± 19.82 | 11.45 ± 18.15 | 10.55 ± 15.25 | 0.05 d | 0.1 |
| Arm symptoms | 30.83 ± 26.73 | 29.26 ± 27.41 | 35.24 ± 26.89 | 16.29 ± 18.71 | 0.03 d | 0.007 |
| Upset by hair loss | 49.16 ± 38.88 | 47.15 ± 36.49 | 48.43 ± 40.25 | 57.77 ± 40.75 | 0.65 d | 0.31 |
| MSPSS | ||||||
| Family | 18.02 ± 2.76 | 18.48 ± 2.55 | 17.90 ± 2.22 | 17.26 ± 4.80 | 0.15 d | |
| Friends | 13.23 ± 5.21 | 14.60 ± 4.47 | 12.31 ± 5.31 | 13.40 ± 6.16 | 0.13 d | 0.2 |
| significant other | 14.45 ± 4.27 | 15.07 ± 3.87 | 13.93 ± 4.41 | 15 ± 4.72 | 0.31 d | 0.2 |
| The total score of social support | ± 9.92 45.71 | 48.17 ± 8.53 | 44.15 ± 9.68 | 45.66 ± 13.31 | 0.12 f | 0.3 |
| Coping strategies | ||||||
| Emotional turmoil | 2.93 ± 0.55 | 2.82 ± 0.52 | 3.03 ± 0.52 | 2.83 ± 0.72 | 0.13 f | 0.11 |
| Reasonable efforts | 4.07± 0.35 | 4.19 ± 0.34 | 4 ± 0.28 | 4.02 ± 0.53 | 0.01 f | 0.02 |
| Avoidance | 3.39 ± 0.55 | 3.40 ± 0.52 | 3.40 ± 0.53 | 3.34 ± 0.69 | 0.92 f | 0.8 |
| Illness adjustment | 150.91 ± 16.29 | 155.58 ± 2.56 | 147.40 ± 1.83 | 153.13 ± 5.12 | 0.03 d | 0.1 |
Average Scores of Quality of Life, Perceived Social Support, and Coping Strategies in the Total Patient and According to Type of Surgery a
4.3. Coping Strategies and Illness Adjustment
Patients who participated in this study used the reasonable efforts coping strategy (4.07 ± 0.35) more than avoidance (3.39 ± 0.55) and emotional turmoil coping strategies (2.93 ± 0.55). Also, patients presented a high degree of adjustment to their illness (150.91 ± 16.29). Patients who underwent lumpectomy used more reasonable effort coping strategies (P = 0.009) and had a higher adjustment to illness (P = 0.01) than patients who underwent a mastectomy. These differences were still significant after adjustment for the covariate. The degree of illness adjustment in patients who underwent lumpectomy than those who underwent a mastectomy was not different (Table 2).
5. Discussion
In this study, Patients had the best score in body image and future perspective and the worst in secual enjoyment and secual function. The symptom indec's highest mean score was related to the side effects of treatment and the discomfort caused by hair loss, consistent with other studies (17). Studies have shown that cancer patients' different life quality areas are not static and change at various stages of the disease, after diagnosis, before and after treatment, and indifferent treatment methods that need special attention (18). Surgery to protect patients' breasts (lumpectomy) is performed in the early stages of cancer, improving body image and life quality (5). In this study, patients who underwent lumpectomy or did not have surgery had a better body image and fewer worries about the future than those who underwent a mastectomy. Previous studies reported that patients who underwent lumpectomies had a better body image than those who ecperienced a mastectomy (19, 20).
On the other hand, in this study, lumpectomy patients showed better secual performance and enjoyment than other patients. A survey in Taiwan showed that the type of surgery affected only functional scores of BR23-FS and that patients undergoing lumpectomy reported better BR23-FS scores than patients undergoing mastectomy (21). A German study also found that patients with conservative breast treatment (BCT) had a better quality of life on most BR-23 scales (22).
Cancer patients use various strategies to deal with the health and psychosocial issues associated with a cancer diagnosis. Evidence showed that most Iranian women use active methods to deal with breast cancer, such as acceptance, religious coping, and planning (23), positively affecting these patients' psychological health and health behaviors, leading women to be more adaptable to their disease (24). In this study, patients mostly used positive coping strategies such as coping with the disease, avoiding negative thoughts about the condition, and having a high illness adjustment rate. Also, lumpectomy patients tried harder to cope with their illness than mastectomy patients and eventually became more adjustable to their illness. A previous study showed no significant difference in the total score of coping skills between the two groups of lumpectomy and mastectomy, and the patients in the mastectomy group used significantly more denial (25).
Patients in this study had high social support, which received the most support from family members, consistent with other studies (26, 27). A previous study showed that cancer survivors receive high social support and family members are the most crucial support source (28). In this study, patients received less support from friends, while another study reported family and friends as the primary sources of support for breast cancer survivors (29). In the current study, women in the lumpectomy group received more social support than women in the mastectomy and non-surgical groups, but this difference was not statistically significant. Received social support may play an essential role in the type of response to surgery. A previous study showed that for women who decided to reconstruct after mastectomy, social support from family and other essential people could play a role in satisfaction with body image after breast surgery (30).
The study had several limitations that should be considered in interpreting the results. Due to the cross-sectional design, quality of life, social support, coping strategy, and illness adjustment were assessed at one point. Therefore, a longitudinal design with a large sample size can investigate these in breast cancer patients on several occasions during treatment and provide more light information.
5.1. Conclusions
Early detection of the disease, patient support, and education programs that teach coping strategies can improve breast cancer women's quality of life and disease adaptation. Providing emotional and social support from patients' family members and treatment team and informing and educating patients to use positive coping strategies can reduce some of the psychological stress resulting from breast cancer diagnosis and treatment.