1. Background
Epidemiological studies have shown an increasing trend in allergies in recent years. Reasons discussed for this phenomenon include a Westernized lifestyle and air pollution (1-4). Recent studies have suggested that environmental pollution has a major role in this phenomenon (5-7), and air pollutants are effective in the formation of allergens, especially pollen allergens (8, 9). They can affect pollen grains during growth periods on the plant or directly using contamination of anthers or pollen grain flight (9, 10).
Several components of air pollution are associated with asthma. In addition to major air pollutants (including sulfur dioxide, nitrogen dioxide, and ozone), diesel exhaust particles (DEPs) and diesel exhaust play an effective role in allergic diseases (11-13). The coarse particulate matter decreased in the atmosphere in the last years, but the amounts of fine particles (less than 2.5 μm; PM2.5), such as DEPs, are still high (14). Diesel exhaust particles are made of a carbon core surrounded by organic chemical components (NO, SO2, NO2, CO, and hydrocarbons) and heavy metals that are deposited (15), of which cadmium (Cd) is an important part.
Cadmium is a well-known environmental pollutant with numerous adverse health effects (16). Cadmium enters the cells using calcium channels or transporters of iron, manganese, and zinc due to its chemical and physical properties similar to these plant nutrients (17). Several studies have examined the effects of Cd on various plants, including the general symptoms of Cd toxicity, such as reduced plant growth (18) and interference with nutrient absorption, transport, and utilization (19, 20).
The study of air pollution and allergy can be enhanced by studying heavy metals since they affect plant proteomes (21, 22). In particular, some heavy metals can change the expression of several proteins related to plant defense (23). Exposure of plants to Cd2+ resulted in the expression of several allergen-like proteins by Arabidopsis thaliana (24). In addition, class I PR-3 chitinases produced in response to heavy metals (25) also have allergenic potency because of their chitin-binding domain (26).
2. Objectives
In this research work, the allergenicity of Petunia pollen grains was evaluated following exposure to Cd as an important part of DEP. Detection of changes in pollen proteins was the other goal because they are associated with pollen allergenicity.
3. Methods
3.1. Plant Materials and Treatments
Petunia plants (Petunia hybrida juss. from the Solanaceae family) were planted in 4 groups in a greenhouse. Forty pots (40 cm height and 40 cm diameter) were filled with soil containing approximately 30% perlite, 20% humus, and 50% loam. Each group included 10 pots, and each pot contained 2 plants. They were kept in stable conditions (25 ± 5°C, humidity 60%, and daylight for 15 hours) in the greenhouse. The experimental groups were classified as follows: Groups treated with (1) 400 μmol/L; (2) 800 μmol/L; and (3) 1200 μmol/L cadmium chloride (CdCl2); as well as (4) control ones treated with water. The plants growing in the pots were treated with Cd solutions 50 days before flowering and continued for two weeks during their pollen formation. The organs of treated and control plants were sprayed with different concentrations of CdCl2 and water, respectively. Each plant was sprayed with about 20 mL of the above solutions every day.
3.2. Pollen Protein Extraction
Protein extracts were performed using immersion of pollen grains in 0.1 M phosphate-buffered saline (PBS) (pH = 7.4) at a ratio of 17% for protein analysis and 8% for injection into mice, using a stirring unit at 5 - 7°C for 4 hours. Centrifugation was performed at 8000 g for 30 minutes (9).
3.3. Electrophoresis
The buffer, including glycerol, sodium dodecyl sulfate, mercaptoethanol, and bromophenol blue, was used as a loading buffer, added to pollen extract samples, vortexed, and then heated in a water bath for 3 - 4 minutes. Pollen extracts (25 μL) and a molecular ladder (15 μL) (Sigma-Aldrich, USA) were run using discontinuous sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (modified Laemmli method) (27). In addition, 4% stacking and 12% resolving gel were performed. Electrophoresis was performed at 80 V twice in a plate gel apparatus (Bio-Rad, USA); then, the bands were stained using 0.2% Coomassie Brilliant blue R 250.
3.4. Animals
Male BALB/c mice (40 - 50 days old) were purchased from Razi Institute, kept in a room at a temperature of 23 - 27°C, and fed a laboratory diet. The mice were treated with intradermal injection of the 40-mL extract (containing approximately 15 mg protein in PBS prepared from control and Cd-treated plant pollen extracts) (28). Aluminum hydroxide (Al(OH)3; in the same amount) was used as an adjuvant in each case. The treatments were repeated weekly for four weeks. They were divided into five groups (n = 6). The first group, as the control group, received PBS and Al(OH)3. The second group was treated with control plant pollen extracts plus alum. Other groups were treated with pollen extracts plus alum of Petunia plants that were treated with three different concentrations of CdCl2 (400, 800, and 1200 μmol/L), respectively.
3.5. Skin Tests
A week after the last sensitization, animals were used for skin tests. Each mouse was injected with 40 mL of the extract (20 mg protein added as an adjuvant) diluted in 0.05 M PBS (pH = 7.4). The negative control was PBS. Skin reactions in the abdominal area were read after 60 minutes from the start of the experiment and measured based on the diameter of the wheel. A positive skin test was considered a wheal diameter of 3 mm or more (29).
3.6. Blood Analysis
Blood samples were obtained directly from the heart (30). The mice were anesthetized with chloroform; then, their blood samples were obtained directly from the heart. The serum from each animal was individually tested for the immunoglobulin E (IgE) reaction.
The serum IgE level was expressed as ng/mL. The blood smears of control mice were compared with those of experimental ones. The numbers of various kinds of blood cells, including eosinophils and neutrophils, were determined in control and experimental animals.
3.7. Statistical Analysis
The results were analyzed using analysis of variance (ANOVA) in SAS 9.1 version (SAS Institute Inc.Cary, NC, USA), as well as the Duncan multiple range test to compare the means of the experimental groups. Data are presented and illustrated as mean ± SE.
4. Results
4.1. Skin Test
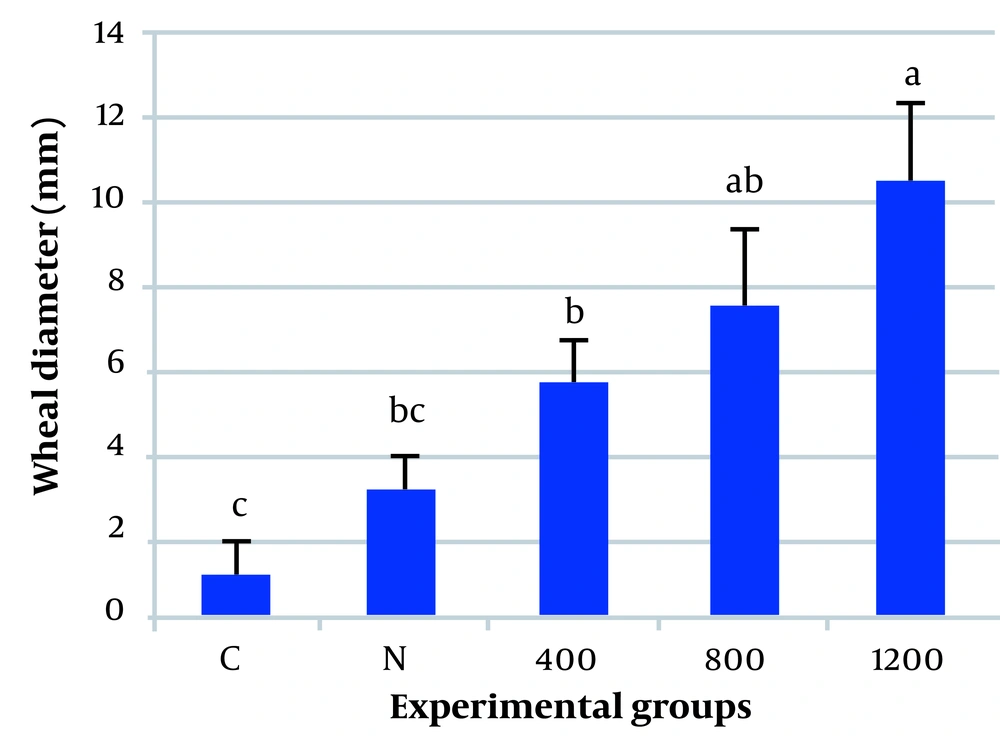
The results of the skin tests showed that pollen extracts prepared from normal plants did not have significant sensitizing effects, but pollen grains from plants exposed to CdCl2 could cause skin reactions (Figure 1). Plants exposed to 1200 μmol/L of CdCl2 showed maximum allergenic activity. Mice receiving this preparation showed a mean wheal diameter of 10.4 mm. Allergic reactions in plants exposed to 400 and 800 μmol/L CdCl2 were also relatively high (average wheal diameters of 5.5 and 7.5 mm, respectively) (Table 1). In contrast, the injection of pollen extracts prepared from untreated plants did not cause obvious skin reactions. Statistical analysis showed that the effect of pollen extracts prepared from plants treated with 1200 μmol/L CdCl2 was about 10 times higher than PBS and about 3 to 4 times higher than pollen extracts of normal plants (P ≤ 0.05).
The mean values of the wheal diameter of the intradermal skin test in 5 experimental groups. The data showed that the skin reaction increased significantly in all groups treated with plant pollen extracts. C, the control group; N, the group treated with the pollen extract of normal plants; numbers 400, 800, and 1200 are the groups treated with pollen extracts of the plants exposed to 400, 800, and 1200 μmol/L of cadmium chloride (CdCl2), respectively. The difference was significant between the 400, 800, and 1200 groups compared with the control group; however, it was not significant between the normal and control groups (P ≤ 0.05). Each column represents the mean ± SE of 6 animals.
4.2. Blood Analysis
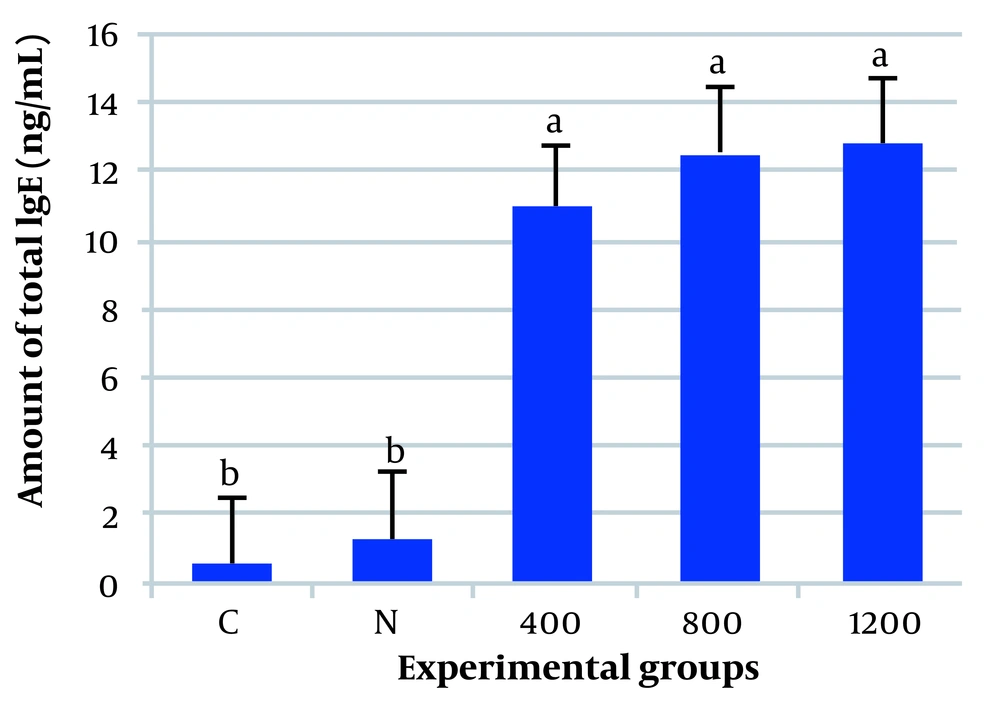
The evaluation of total IgE levels in blood samples indicated that all pollen extracts of the plants were able to increase the levels of IgE (Figure 2). The data and results showed that IgE increased considerably in animals injected with the pollen extracts of Cd-exposed plants. The quantity of IgE in these animals was about 20 - 25 times more than the mice treated with PBS only, as well as 9 - 10 times more than the group treated with non-polluted pollen.
The amount of the immunoglobulin E (IgE) (ng/mL blood) of the control and experimental mice. Cadmium-exposed plant pollen extracts caused an increase in blood IgE as allergic characteristics. C, the mice that were injected with saline; N, the mice that received non-polluted pollen; numbers 400, 800, and 1200 are the groups treated with the pollen extract of the plants exposed to 400, 800, and 1200 μmol/L of cadmium chloride (CdCl2), respectively. Each column represents the mean ± SE of at least 6 animals.
4.3. Protein Analyses
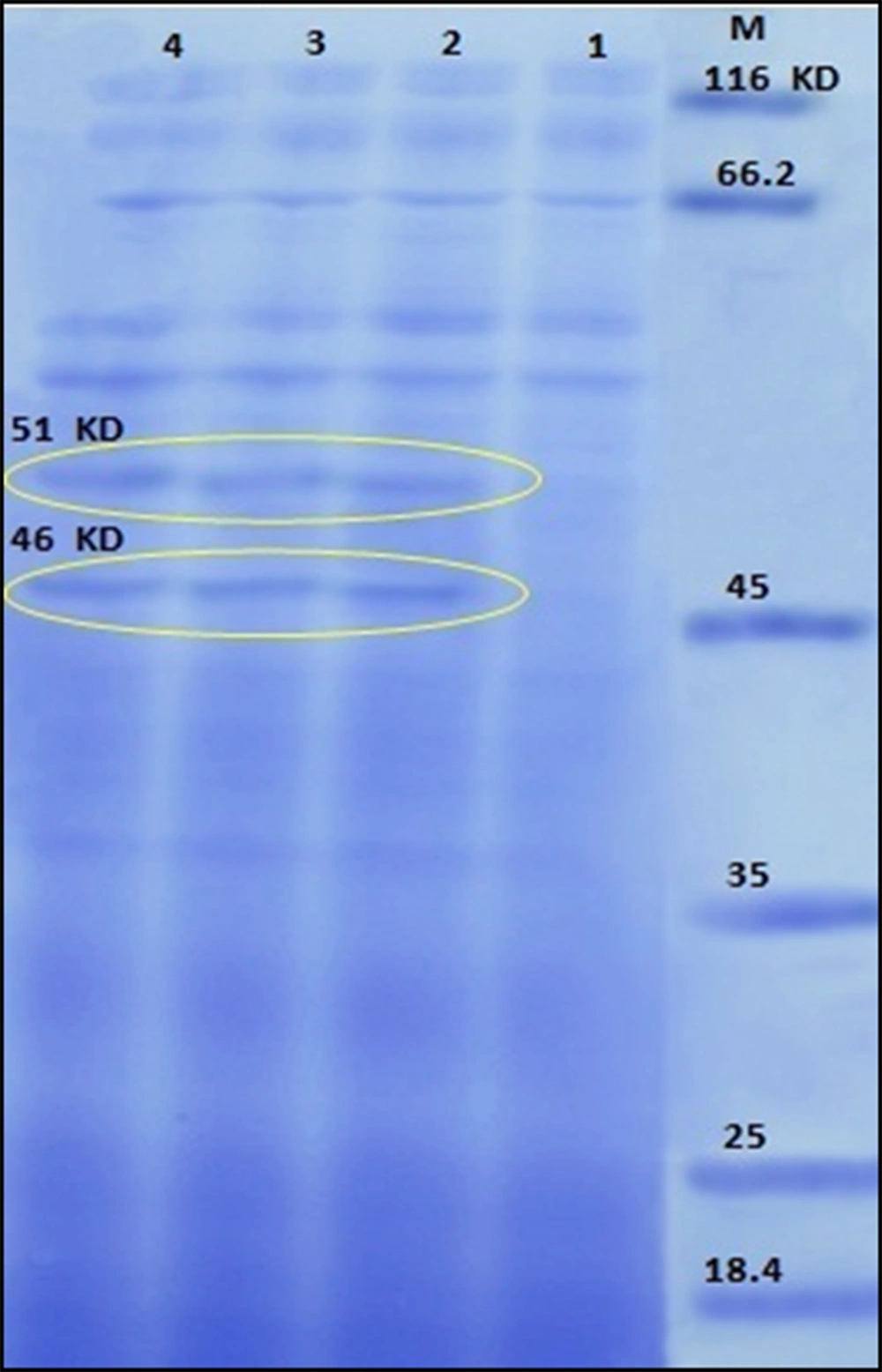
The pollen protein profiles of normal plants and those exposed to Cd ranged in molecular weight from 15 to 120 kDa (Figure 3). SDS-PAGE showed that the pollen grains of plants exposed to different concentrations of Cd have different bands that were not observed in the extract of normal plants. They showed 2 extra bands (46 and 51 kDa).
The protein profile of experimental pollen. The pollen of the plants treated with cadmium chloride (CdCl2) has 2 extra bands (46 and 51 kDa) that are different from normal pollen. M, molecular weight standard markers; 1, the pollen grains of normal plants; 2, 3, and 4, the plants that were exposed to 400, 800, and 1200 μmol/L of cadmium chloride; IgE, immunoglobulin E
5. Discussion
The allergenic potential of pollen of plants treated with Cd and normal plants was investigated using induction wheals. The total IgE was evaluated in the blood of experimental mice. Observation and serological tests showed the mean diameter of wheals and IgE level in animals treated with normal plant pollen extract were close to the levels of the control group. This means that P. hybrida is not a real allergen species. However, the results clearly showed that Cd could induce the allergenic potential of pollen grains. On the other hand, the results of SDS-PAGE analysis for soluble proteins (Figure 3) showed different bands in the pollen grains of treated and untreated plants. It seems that CdCl2 treatment can affect the pollen development process. Therefore, we can assume that this allergenic potential is apparently due to the formation of new pollen allergen proteins (46 and 51 kDa). This finding is consistent with some previous studies (31-34) and inconsistent with some others (1, 35).
Scientific evidence shows that pollen in heavily polluted areas expresses more proteins described as allergenic (36, 37). Some previous reports have shown that the electrophoresis profiles of pollen collected from polluted and non-polluted regions are similar without any significant differences (30, 38). However, the studies of pollen protein analysis (from polluted and non-polluted areas) show conflicting results.
In recent years, several studies have been conducted on the effects of environmental pollution on the allergenic power of pollen. Air pollutants have been shown to adhere to pollen surfaces (39), interact with pollen allergens, and act as adjuvants to the immune system, thus enhancing the allergenic properties of pollen (34, 36).
Allergenic sources derived from pollen are called Ubisch bodies. They are spherical and cause the growth of the pollen exine. They are visible in the anther of different plant taxa. Allergenic proteins may also be a major part of the structures. Some non-biological suspended particles (e.g., DEPs) act as carriers and react with them, as do other pollen-derived paucimicronic particles (37, 39). On the other hand, stronger Ig-mediated responses to such particles and inflammation of the respiration system may increase the frequency of allergic diseases in air-polluted areas (39).
5.1. Conclusions
The frequency of allergies has increased dramatically in recent years. Air pollution seems to be responsible for this phenomenon. Diesel exhaust particles are the most important air pollutants that can increase allergy frequency in polluted cities. Considering that Cd is an important part of DEP, we can conclude that it is an effective agent in the formation of detoxification proteins that can act as new allergens. It is possible that Cd can act as an allergen and be an effective agent that requires the formation of detoxification proteins.