1. Background
In recent decades, a shift in living conditions has led to a rise in obesity across many human societies (1). Individuals who are overweight or obese often experience fatty liver disease, a chronic liver condition characterized by lipid accumulation in liver cells, typically resulting from more than 5% fat content in the liver. Fatty liver disease can progress from simple steatosis to steatohepatitis, eventually leading to advanced fibrosis and cirrhosis (2). The transport and movement of FAs across the cell membrane are facilitated by three groups of sarcolemma transporter proteins. In muscle tissue, these include the fatty acid (FA) binding protein in the plasma membrane (FABPpm), a family of fatty acid transfer proteins (FATPs), and fatty acid translocase (FAT/CD36), the latter being a homolog of human CD36. Among these, FAT/CD36 plays the most significant role in the rate of FA transfer (3). After lipid digestion, the transport of short-chain FAs occurs through simple diffusion, whereas the transport of long-chain FAs is managed by various transporters in the enterocyte membrane, including CD36, FABPs, and FATP1-6. Within the cytoplasm of enterocytes, FAs are thioesterified by acyl-CoA synthetase-1 to form acyl-CoA, which is then used with monoglycerides to synthesize triglycerides (TG) (4). CD36, a plasma membrane component of hepatocytes, plays a crucial role in lipid transfer, facilitating the transport and transfer of FA across the mitochondrial membrane and into hepatocytes (5). Changes in CD36 expression have been shown to significantly impact FA accumulation, where a reduction in CD36 leads to decreased secretion of VLDL, reduced release of TG and apoB, and an increase in prostaglandins D2, F2 and E2, ultimately resulting in steatosis in rats (6). Additionally, the adoption of a high-fat diet, which induces NAFLD, has been linked to an increased expression of CD36 (7).
Adopting a low-fat diet has been shown to decrease the expression of CD36, resulting in a significant reduction of FA uptake in hepatocytes, even with an increase in plasma fat (8). Additionally, FABP, in conjunction with CD36, facilitates the transport of long-chain fatty acids (LCFA) into liver cells. FABP, with its high affinity for FAs, can transport two FA molecules across the membrane (9). The impact of exercise training on FA transporter protein levels has yielded mixed results. Burgomaster et al. noted that 6 weeks of high-intensity training (HIT) exercises increased muscle oxidation capacity by 35% (increase in COX IV content within the first week) but had varying effects on glucose, lactate, and FA transporters. The content of GLUT4, MCT1, and MCT4 significantly increased and remained elevated above baseline levels even after 6 weeks without training. However, neither training nor the lack of training significantly altered FAT/CD36 and FABPpm content (10). Talanian et al. observed a significant increase in FABPpm levels in the vastus lateralis muscle after 7 weeks of HIT training, while the FAT/CD36 protein content showed no significant change post-training (11). Conversely, Seldin et al. found that two weeks of voluntary treadmill exercise significantly increased the expression of FAT/CD36 and FABP4 in adipocytes, although there was no significant increase in FABP1 levels in adipocytes (12).
2. Objectives
Given these mixed findings, along with the absence of studies on FA transporters in liver tissue and the lack of research involving high-intensity interval training (HIIT), we aimed to investigate whether 8 weeks of intense interval training affects the levels of hepatic lipid transporter proteins (LFABP and CD36) in rats with fatty liver.
3. Methods
3.1. Animals
This study employed an applied, semi-experimental, and double-blind experimental design. Thirty 10-week-old rats with an average body weight of 165.4 ± 31.3 g were sourced from the laboratory at Jundishapur University, Ahvaz. Throughout the research, the rats were housed in specialized fiberglass cages under controlled conditions, including 12 hours of darkness and light, a temperature of 23 ± 1°C, and humidity of 51 ± 3%. It's important to note that each cage housed three rats.
Throughout the study, the rats' water and food supplies were monitored daily, ensuring they had access to standard, species-specific provisions. Additionally, their cages were cleaned thoroughly twice a week.
3.2. Method
After a week of acclimatizing to the laboratory setting, thirty rats were randomly assigned into two groups: A sham group (10 rats) and an experimental group (20 rats, divided into two subgroups: Fatty liver and fatty liver + exercise). Animals in the sham group were fed standard food for 16 weeks, whereas those in the experimental group received a high-fat diet under similar conditions. The sample size for each group was calculated using MedCalc version 10.0.2.0, based on a 5% significance level and a test power of 0.8, resulting in approximately 10 subjects per group.
Following the initial 16-week nutritional intervention, blood samples were collected from the tails of all animals on the high-fat diet to determine plasma ALT levels, a primary indicator of fatty liver.
Subsequently, animals in this group were randomly divided into two equal subgroups: A control group and a HIIT training group. While both subgroups continued on the high-fat diet, the HIIT training group underwent a HIIT protocol for 8 weeks (5 days per week). In contrast, the control group only maintained the diet, receiving high-fat food without engaging in any exercise program.
The exercise protocol comprised 8 weeks of interval training featuring stages of high and low intensity:
- High-intensity stages involved 2 minutes at 75% of maximum speed during the first week, increasing to 80% in the second week, 85% in the third week, and reaching 90% in the fourth week, which marked the conclusion of the training period (Table 1) (13).
- Low-intensity stages consisted of 2 minutes at 30% of maximum speed from the first to the third week and were adjusted to 20% of maximum speed from the start of the fourth week until the end of the training period (Table 1) (13).
| Exercise Protocol | First Week | Second Week | Third Week | Fourth Week | Fifth Week | Sixth Week | Seventh Week | Eighth Week |
|---|---|---|---|---|---|---|---|---|
| Intense training intensity (percentage of maximum speed) | 75 | 80 | 85 | 90 | 90 | 90 | 90 | 90 |
| Intensity of slow training (percentage of maximum speed) | 30 | 30 | 30 | 20 | 20 | 20 | 20 | 20 |
| The number of practice sessions | 2 | 4 | 6 | 8 | 8 | 8 | 8 | 8 |
High-intensity Interval Training Protocol
3.3. Statistical Analysis
Descriptive statistics, including means and standard deviations, were utilized to present the data and create tables. The Shapiro-Wilk test was applied to assess the normality of the data, while Levene's test was conducted to examine the homogeneity of variances. An independent t-test was used to compare group means in the pre-test and post-test. To explore the differences between groups, one-way analysis of variance (ANOVA) and Bonferroni's post hoc tests were employed. All data were analyzed using SPSS-22 software, with a significance level set at 0.05.
4. Results
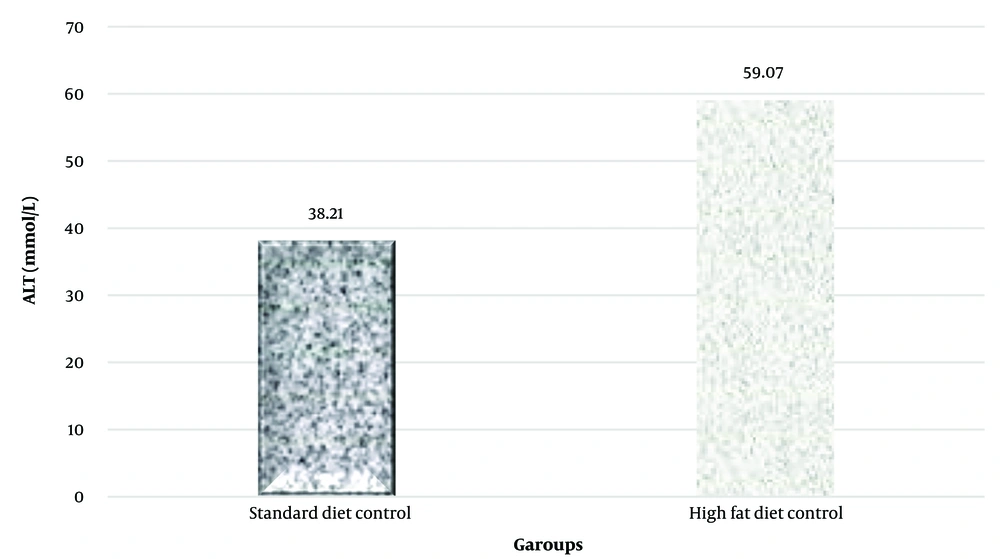
In this study, the plasma levels of the alanine aminotransferase (ALT) enzyme were examined to investigate the development of non-alcoholic fatty liver. The t-test results indicated that a high-fat diet significantly increased plasma ALT levels (P = 0.001) (Table 2 and Figure 1).
| Variable and group | No. | Mean a | Main Difference | t | P-Value |
|---|---|---|---|---|---|
| Alanine aminotransferase, mmol/l | 20.86 | 9.73 | 0.001 | ||
| Standard diet control | 10 | 38.21 ± 4.15 | |||
| High-fat diet control | 10 | 59.07 ± 9.42 |
Independent t-test Results to Investigate the Effect of High-fat Diet on ALT Plasma Levels
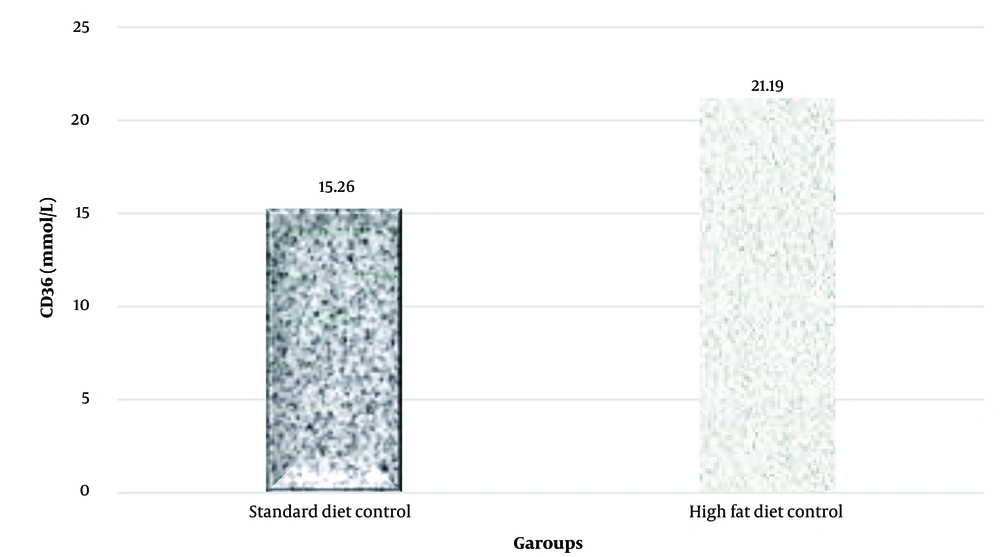
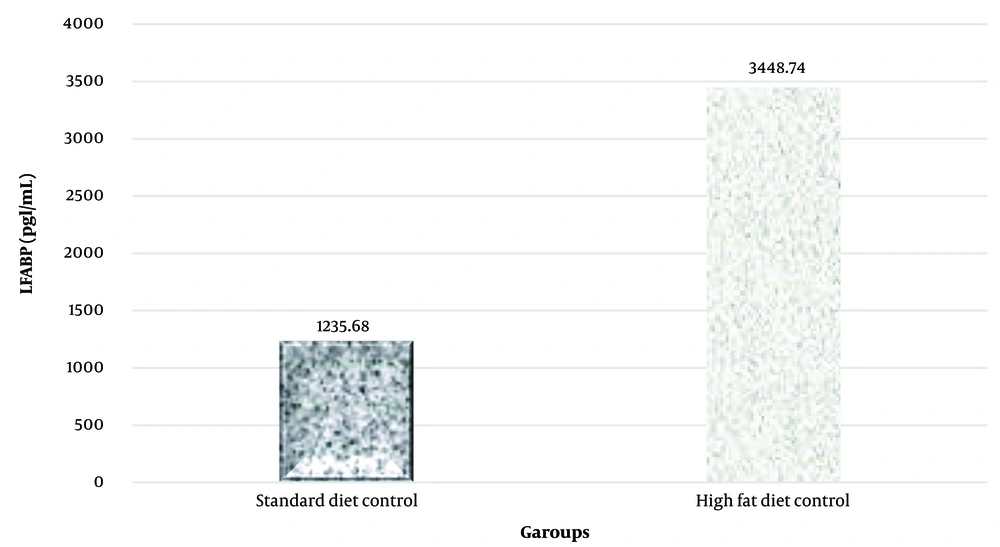
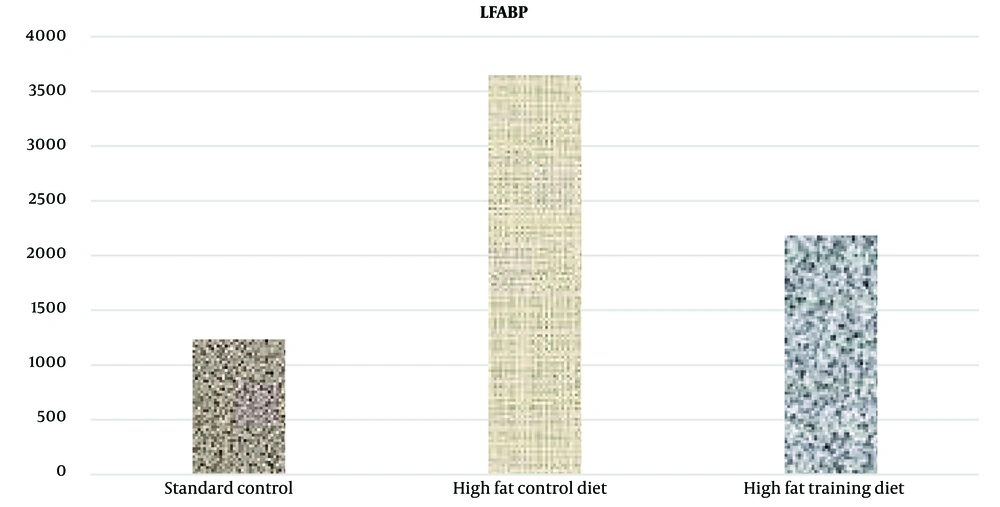
A notable increase in CD36 and LFABP protein levels in liver tissue was observed in the high-fat diet group (P = 0.023 and P = 0.001, respectively) (Figures 2 and 3).
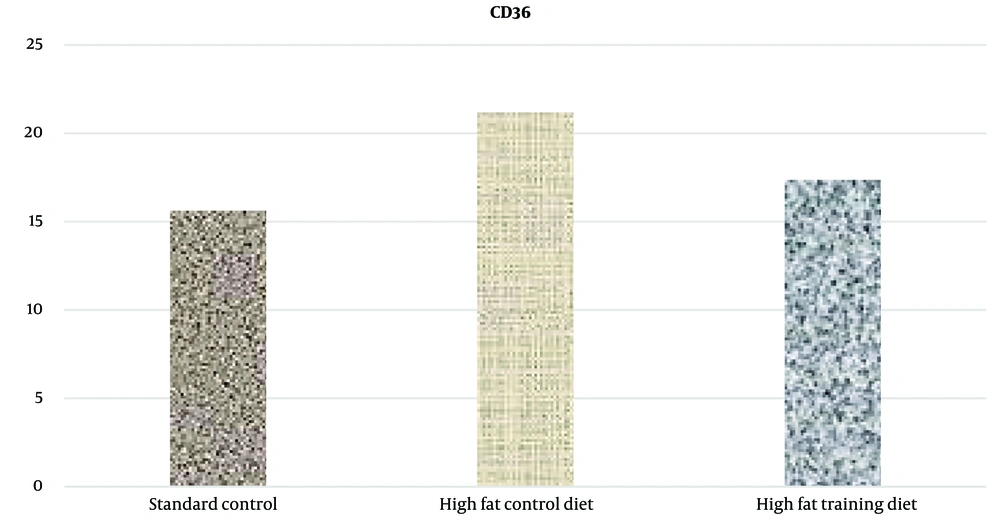
Additionally, the findings demonstrated that HIIT significantly reduced CD36 and LFABP protein levels in liver tissue in rats (Figures 4 and 5).
5. Discussion
ALT is recognized as a more specific and sensitive marker for liver cell damage compared to AST. Typically, the increase in ALT levels during hepatocellular injury exceeds that of AST due to ALT's longer half-life (14, 15). The use of a high-fat diet in this study led to inflammation and liver damage in rats, with a consequent increase in plasma ALT levels, indicating the development of non-alcoholic fatty liver. The research findings revealed that liver CD36 and LFABP protein levels significantly increased by the end of the 16th week. Thus, the induction of non-alcoholic fatty liver resulted in a significant rise in liver CD36 and LFABP protein levels. Hepatic expression of FAT/CD36 is usually low but increases in rodents with fatty liver (16). Furthermore, a period of high-fat diet consumption leads to non-alcoholic fatty liver disease (NAFLD), accompanied by heightened CD36 expression (17, 18).
Fatty acid-binding proteins (FABPs) play a crucial role in the transport and metabolism of FAs. Indeed, FABPs facilitate the utilization and consumption of dietary fats, and in tissues where FA metabolism, absorption, and storage are significant—such as the intestine, liver, adipose cells, and muscle cells—the quantity of FABPs increases alongside the heightened use of FAs (19).
This study's findings indicate that 8 weeks of HIIT significantly lowered the levels of liver CD36 and LFABP proteins in rats subjected to this training regimen compared to those in the high-fat diet control group. As observed in this research, a possible explanation for the reduction in LFABP levels could be the decrease in body weight and subsequent fat reduction in the training group relative to the control group. Moreover, according to Lira et al., the reduction in LFABP levels following exercise was attributed to a decrease in intracellular triacylglycerol (20). Additionally, considering that testosterone levels rise after prolonged exercise training (21, 22), and given that increased testosterone is associated with reduced LFABP levels (23, 24), it is plausible that another contributing factor to the decreased liver LFABP values is the elevation of testosterone levels due to long-term exercise training (25).
The inconsistency of the results of this study with other research that reported an increase in LFABP levels can be attributed to the use of non-fatty liver test samples and a focus on the effects of exercise training on diabetic samples in those studies. It appears that the enlargement of fat cells due to weight gain and obesity correlates with an increased expression of FABPs and their release into the bloodstream (26, 27). Consequently, the reduction in FABP levels observed in diabetic samples might be due to the degradation of muscle tissue and fat, which are primary sources for the production and secretion of FABPs (28).
Non-alcoholic fatty liver is often a result of sedentary behavior and a high-calorie diet. Thus, according to the findings of this research, individuals with NAFLD should, in addition to modifying their diet and reducing calorie intake, engage in suitable exercise training to mitigate the complications and effects of this condition. HIIT, in particular, is recommended.