1. Background
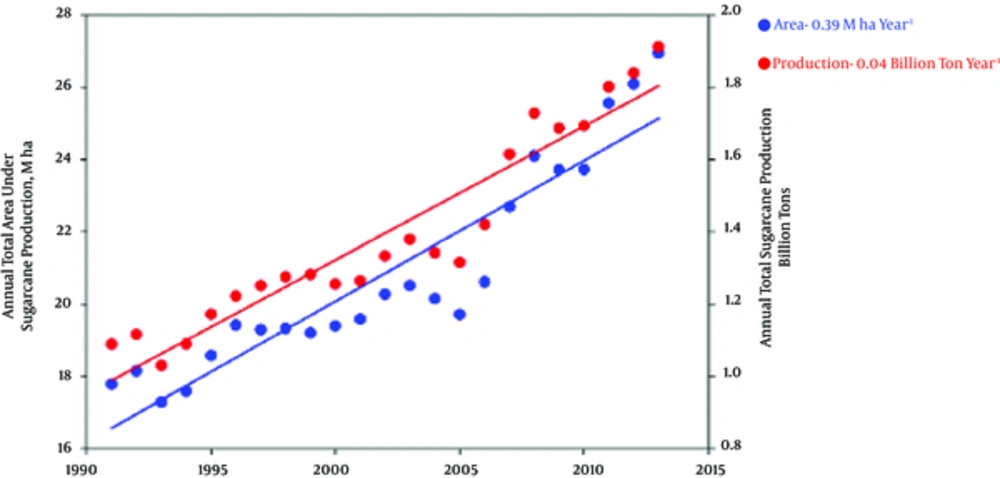
The area of sugarcane cultivation increases each year (1). Based on the FAO report, it was 0.39 (Mh) in 1990 and it increased to 26.94 (Mh) in 2013 (Figure 1).
Global Annual Sugarcane Area and Production During the Period from 1990 to 2013 (2)
The massive production of ethanol from sugarcane has produced vinasse as by-product (3, 4). After molasses is converted into alcohol and its other derivatives, Vinasse must leave the factory as waste material (5). The main concern with the environmental impact associated to practices of vinasse application in soils is the potential contamination of rivers and ground water (6).
Concentrations of nutrients in Vinasse vary depending on the conditions of the factory and the quality of the molasses or syrup. Vinasse is an organic material, which consists of 90% water, acidic (pH 3.5 - 5), dark brown slurry (3), nitrogen (1.4%) and potassium (1.16%), and has the electrical conductivity of 39 dS/m (5).
The high percentage of organic and inorganic materials in wastewater of ethanol production plants makes them 1of the most troublesome types of industrial wastewater. Moreover, presence of toxic materials such as aromatic compounds (especially phenols), low pH values, and high percentage of mineral materials add to the difficulty of treating wastewater of these factories. Considering the stable brown color of Vinasse, the removal of which is another problem in the treatment of these wastewater, the issue of treating wastewater of ethanol production factories has turned into a global problem (7). Heavy metal pollution has progressively become a widespread problem with increasing industrial and agricultural activities (8, 9). It is responsible for causing adverse effects on human health through food chain contamination (9, 10).
The accumulation of heavy metals in natural and soil were identified as one of the major hazardS to humanity and may affect the functions of ecosystems and degrade soil quality (11-13). Metals in soils compromise crop yield and growth and poses a risk to animal health due to consumption of contaminated soil and vegetation. Metal also possesses a hazardous effect to human health because of the consumption of contaminated soil, plant produce, and water from surface or underground resources (11).
Lead (Pb) contamination of soil is a threat to human health and ecosystems. Several studies have shown that for every 1000 mg kg-1 of Pb in the soil, the blood Pb in children can be elevated by 1 - 5 μg dL-1 (14).
Cadmium has no known useful biological function for human beings, animals, or plants, however, it produces severe diseases and disorders, this ion being one of the most hazardous materials with strong phytotoxic effects (15). Environmental contamination by cadmium is important because high levels of this metal represent a serious problem to human health, living resources, and ecological systems (16). The Cd pollution of water bodies is less widespread compared to mercury or lead, however, its high toxicity produces severe hazardous effects on human beings (17). Cd is more available than other heavy metals to migrate to deeper soil layers or to underground water by leaching (18, 19).
There is an immediate and critical need to ensure safe soils for society (14). Leaching is the most common method used for extracting metals. which involves the application of a leaching solution onto mounds of soil with drainage support. Another alternative for managing contaminated soils that is the least desirable is the removal of soil and its disposal in a landfill. This method is not preferred because it only relocates the contaminated soil without addressing the contamination problem. Soil flushing and washing are recognized techniques for heavy metal leaching. However, soil nutrients may also be released during the process of remediation (20).
Zeolite and vermiculite have wide applications such as water adsorption, wastewater treatment, molecular sieves for plants, air filtration, etc. They are suitable materials for reducing heavy metals and are effective in removing heavy metals as well as selective adsorption, dehydration rehydration, cation exchange capacity, and catalysis properties (21). Pb, Cd, and Hg, which are typical toxic heavy metals, could be tacked by zeolite. The application in agriculture is suitable due to their ion exchange capability without major changes in structure (22).
There are wide studies regarding zeolite to absorbing heavy metals from industrial, urban, and agricultural wastewater. Wen et al. (2016) showed that Zeolite is the best absorbent for Pb, Cu, Zn, and Cd removal from wastewater (23).
2. Objectives
Over the years, several methods for remediation of soils contaminated with heavy metals have been proposed and tested. Leaching is the most common method used for extracting metals (11). The aimed of this research is reduce lead and cadmium in Vinasse with uses of Zeolite and Vermiculite by soil column.
3. Methods
This research was conducted to study the ability of zeolite and vermiculite in reducing concentrations of cadmium and lead in waste water of an ethanol-producing factory, and to investigate their effects on soil physical and chemical characteristics under natural conditions in a clay loam soil. Soil samples were taken from around the Vinasse storage pond at the Debal Khazaei Agro-industrial Co., which is located 25 kilometers from Ahvaz on the Ahvaz-Abadan road. Soil samples weighing approximately 1 kg were taken from the depth of 30 cm at different spots that were 1 meter apart. The sample was transferred to the laboratory, air dried, and analyzed to determine its initial characteristics. Vinasse samples were taken at its outlet from the last Vinasse storage pond, and 20-liter containers were used to transfer and store wastewater samples.
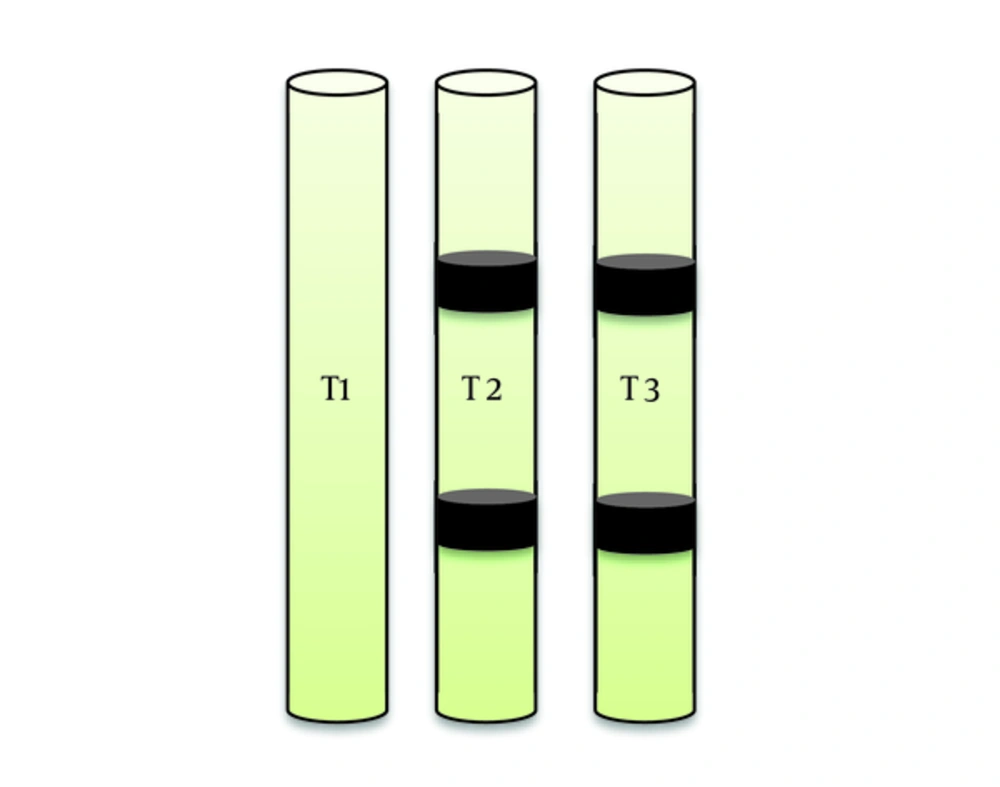
To evaluate the potential of Vermiculite and Zeolites in refinement of metal pollutants cadmium and lead, 2 irrigation periods by Vinasse and 3 treatments were conducted. The experiment was performed within columns with diagonal of 9 cm and height of 100 cm in 3 treatments and 3 replications with 1 blank. In treatments 2, 3 used Zeolite and Vermiculite in 25 and 50 cm soil depth (Figure 2).
Table 1 shows the treatments applied in this research conducted for potential assessment of zeolite and vermiculite in removing metal pollutants from wastewater.
| Treatments | Component |
|---|---|
| 1 | Soil + Vinasse |
| 2 | Soil + Vinasse + Zeolite |
| 3 | Soil + Vinasse + Vermiculite |
Treatments
Surface irrigation (flooding) with the volume of 2.3 liter was conducted every 5 days. First, the soil of the region was irrigated by Vinasse up to the saturation level. Second, the soil of the region with 2 layers of Zeolites in the depths of 25 cm and 50 cm was irrigated by Vinasse up to the saturation level. Third, the soil of the region with 2 layers of Vermiculites (same depths as treatment two) was irrigated by Vinasse up to the saturation level.
After soil arrived to field capacity, sampling in the depths of 15 - 25 and 40 - 50 cm of soil columns and drained water from the bottom of the soil columns were collected 24 hours after each irrigation and samples were taken to laboratory to perform chemical analyses.
The hydrometer method was used to determine soil texture, the paraffin-coated clod method for bulk density, the pycnometer method for particle density, an electrical conductivity (EC) meter for EC of saturated soil paste, and a pH meter for pH of saturation extract (24).
Lead and cadmium were measured employing the method of wet digestion using nitric acid, and the prepared samples were then put in an atomic absorption spectrometer (an Elmer Perkin model) to read concentrations of lead and cadmium (25).
Drainage water samples were taken during 2 irrigation intervals (separately and 24 hours after each irrigation interval from each soil column) and transferred to the laboratory to perform tests and determine their chemical quality. Diagrams were drawn employing Excel.
4. Results
The study region, the Dabal Khazaei Agro-industrial Co., has a latitude of 31°8’ north, longitude of 48°35’ east, and altitude of 7 m. The soil in the study region has a mainly clay loam texture, and is poor in the organic matter and mineral elements due to continuous cultivation of sugar cane (30).
The results of initial analyses of soil samples are presented in Table 2. Soil Pb and Cd is 6.20 and 0.42 (mg.kg-1), respectively.
| EC, ds.m-1 | pH | Sand, % | Silt, % | Clay, % | Ρb, g.cm-3 | Ρs, g.cm-3 | Pb, mg.kg-1 | Cd, mg.kg-1 | |
|---|---|---|---|---|---|---|---|---|---|
| Results | 155.50 | 6.61 | 22.50 | 46.00 | 31.50 | 1.31 | 2.68 | 6.20 | 0.42 |
Soil Initial Properties
The results of initial analyses of the collected Vinasse samples are presented in Table 3. Cd and Pb in Vinasse is 7.11, 46.20 (mg/kg), respectively. These samples were saline (had high EC values), had acidic pH values, and were contaminated with lead and cadmium.
| Cd, mg.kg-1 | Pb, mg.kg-1 | pH | EC, ds.m-1 | |
|---|---|---|---|---|
| Results | 7.11 | 46.20 | 4.90 | 78.10 |
Some of the Vinasse Properties
4.1. Soil Cadmium and Lead Concentrations
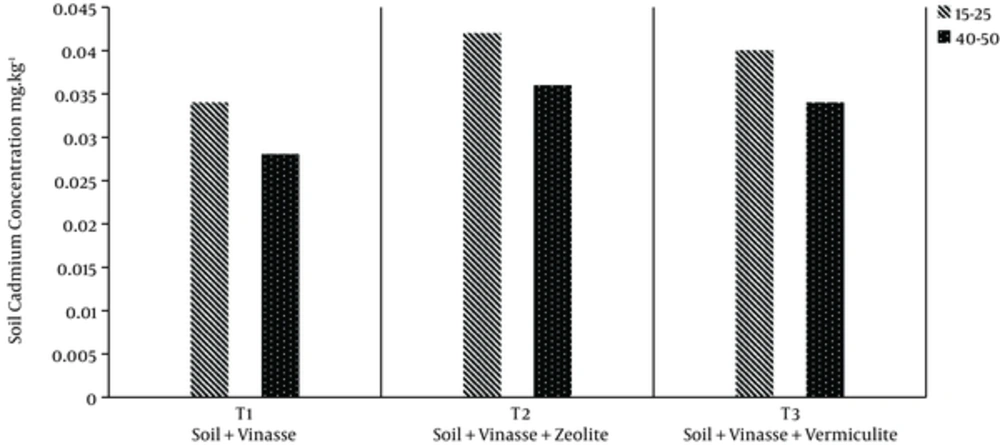
The extents of reduction in soil cadmium concentration at the 2 depths were decreased (Figure 3). The values in treatments T1, T2, and T3 were 0.034, 0.042, and 0.040 in the first depth and 0.028, 0.036, and 0.034 mg/kg in the 2nd depth, respectively.
The highest soil cadmium concentration is observed in the 2nd treatment (zeolite).
Therefore, the application of adsorbents in Treatments T2 and T3 was not effective in decreasing cadmium concentration compared to treatment T1. These results are contrary to those found by Suubramanian and Gupta (2006).
The soil under study was not contaminated with cadmium, however, addition of Vinasse to it could result in its contamination by cadmium. Most of the cadmium was probably adsorbed in the surface layer of the soil, and cadmium concentration declined with increases in soil depth in these treatments.
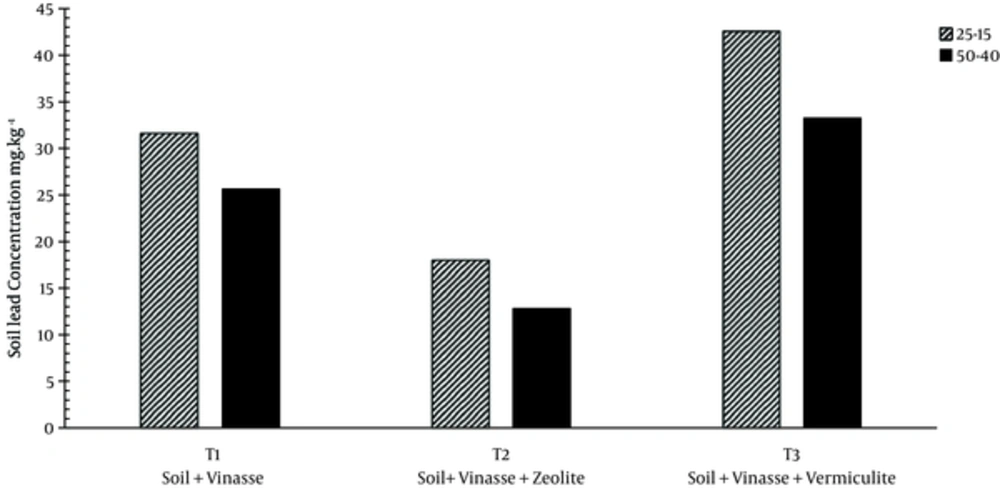
The lead values in treatments T1, T2, and T3 were 31.36, 18.01, and 42.51 in the 1st depth and 25.62, 12.81, and 33.25 mg/kg in the 2nd depth, respectively (Figure 4).
Therefore, applying zeolite was effective in decreasing lead concentration in the drainage water in treatment T2 compared to treatments T1 and T3. However, application of vermiculite had no effect on lead adsorption, which is in agreement with results from Awan et al. (2003) who reported on the removal of the 4 heavy metals lead, chromium, copper, and nickel.
4.2. Cadmium and Lead Concentrations in the Drainage Water
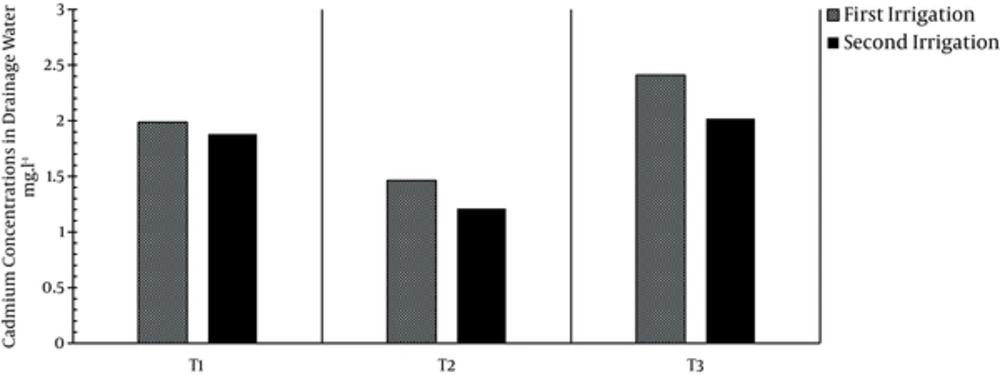
Concentrations of cadmium in the drainage water related to treatments T1, T2, and T3 were 1.99, 1.46, and 2.41 in the 1st irrigation and 1.89, 1.21, and 2.02 in the 2nd irrigation (Figure 5). Application of zeolite was effective in reducing cadmium concentration in the drainage water in treatment T2 compared to treatments T1 and T3, and zeolite adsorbed cadmium the most; however, vermiculite was not effective in adsorbing cadmium. These results conform to those reported by Forber et al. (2005).
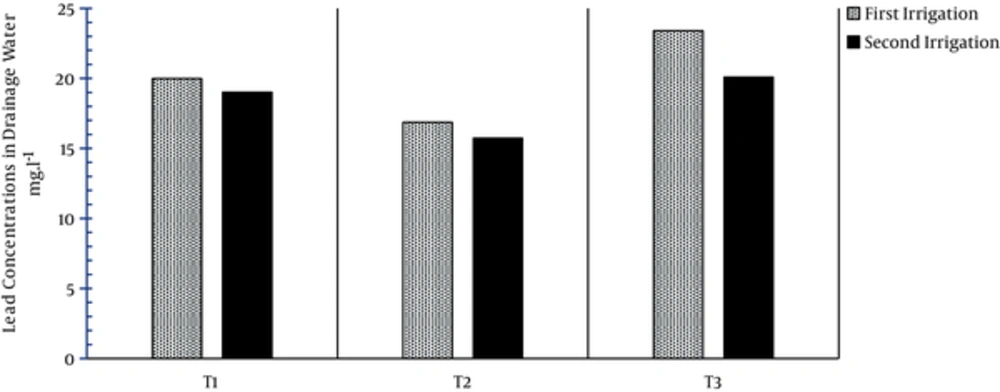
The concentrations of lead in the drainage water related to treatments T1, T2, and T3 were 19.98, 16.87, and 23.4 in the 1st irrigation and were 19.01, 15.73, and 20.09 in the 2nd irrigation (Figure 6). Application of zeolite was effective in reducing the concentration of cadmium in the drainage water in treatment T2 compared to treatments T3 and T1. These results are in agreement with those reported by Awan et al. (2003).
5. Discussion
The area of sugarcane cultivation increases each year. Concentrations of nutrients and heavy metals in Vinasse vary depending on the conditions of the factory and the quality of the molasses. The accumulation of heavy metals in natural and soil were identified as one of the major hazard to humanity and may affect the functions of ecosystems and degrade soil quality. There are wide studies about zeolite to absorbing of heavy metals from industrial, urban, and agricultural wastewater. Wen et al. (2016) show Zeolite is the best absorbent for Pb, Cu, Zn and Cd removal from wastewater (23). Akkajit P. et al. (2013) in research of fractionation of Cd and Zn in Cd-contaminated soils amended by Vinasse, he shows the observed reduction in exchangeable Cd (BCR1) in the amended soils at the 3 % (w/w) application rate, the low total metal concentrations, and the significant amount of essential plant nutrients (N, P, and K) within these waste products highlight the benefits of amending metal-rich soils with them (27). Muñoz SV et al. (2014). was used two types of dense commercial pyrogenic silica nanoparticles with different chemical groups on the surface to adsorb cadmium ions: Aerosil A130VS and R972. The concentration of cadmium ions was reduced: from 109 to 0.01 ppm for A130VS, from 138 to 1.44 ppm for R972, and from 123 to 0.005 ppm for sol–gel (17). Liu J et al. (2016) indicated that weathered coal humic acid and simulated acid rain significantly decreased the available surface soil Pb concentrations. The removal of Pb was efficient, particularly at the 1000 mg.kg−1 Pb pollution level, with a maximum decrease of 85.8 % (9). Najafi, P et al. (2016) in three treatments included natural zeolite, perlite and vermiculite were used in pitcher irrigation show the heavy metals especially Pb and Zn were increased value 75 and 80 times compared with initial values, respectively (22). Yilmaz (2016) was used zeolite for remove lead and nickel in synthetically solution. He reported that the Zeolite is suitable for adsorb both heavy metals and he measured the maximum capacity adsorption of lead and nickel as 682 and 122mg/kg after 60 mint in one atmosphere pressure, respectively (28). Zeolite was used to remove heavy metals in wastewater produced of graphic industry by Zanin. The most adsorption was obtained for Iron (III), Cupper and chromium(III) as 95.4%, 96% and 85.1%, respectively (29) The results of all these studies concur with those of our study. Over the years, several methods for remediation of soils contaminated with heavy metals have been proposed and tested. Leaching is the most common method used for extracting metals. Addition of Vinasse to soil in treatments T1, T2, and T3 increased soil lead concentrations compared to the soil in the region. Application of zeolite was effective in reducing cadmium concentration in the drainage water in treatment T2 compared to treatments T1 and T3, these results are in agreement with those reported by Awan et al. (2003) and Forber et al. (2005). Zeolite adsorbed cadmium the most, but vermiculite was not effective in adsorbing cadmium. These results are contrary to those found by Suubramanian and Gupta (2006) because it is neutral and application of adsorbents in treatments T2 and T3 was not effective in decreasing cadmium concentration compared to treatment T1. The soil under study was not contaminated with cadmium, but addition of Vinasse to it could result in its contamination by cadmium. Most of the cadmium was probably adsorbed in the surface layer of the soil, and cadmium concentration declined with increases in soil depth in these treatments. In the current research the concentration of lead in drainage water in all three treatments T1, T2, and T3 decreased compared to the sores Vinasse, but the maximum reduction in lead concentration in the drainage water was that of the treatment in which zeolite was applied. Cd and pb in drainage water is more than WHO's for Drinking-water Quality (Cadmium and lead 0,003 to 0,01 mg/l respectively) (30). Vermiculite was not effective in adsorbing lead compared to the treatment in which no adsorbent was used. Addition of Vinasse to the treatments T1, T2, and T3 increased cadmium adsorption from the soil in all three treatments, and zeolite and vermiculite were not effective in decreasing soil cadmium concentration in the soil in these treatments. Concentrations of lead and cadmium in the drainage water in all three treatments declined compared to the sores Vinasse, but the minimum concentrations of cadmium and lead were observed in the wastewater of the treatment in which zeolite was applied compared to the treatments of applying vermiculite and the treatment of not applying any adsorbent. Addition of Vinasse to soil in all treatments increased soil lead concentrations compared to the soil in the region. The concentration of lead in drainage water in all three treatments decreased compared to the sores Vinasse. Vermiculite was not eff