1. Background
Irvingia gabonensis, commonly known as African mango or wild mango, is a tree species native to West Africa. It has been traditionally used for various medicinal purposes, including the treatment of diarrhea, dysentery, and diabetes (1). The bioactivity of Irvingia gabonensis has gained significant attention in recent years due to its potential therapeutic properties.
Both the seeds and fruits are well consumed, the seeds are particularly valued for the high fat and protein contents of the nut. The fat is extracted from the seeds for soap and candle making. A relish made from the Dika nut is customarily eaten with plantains. The nuts are used principally for food and oil and in weight loss supplements (2).
Brine shrimp lethality bioassay is easily mastered, costs little, and utilizes a small amount of test material. This provides a front-line screen that can be backed up by more specific and expensive bioassays once the active compound has been isolated. It is evident that brine shrimp lethality bioassay is predictive of cytotoxicity and bioactivity (3). The brine shrimp lethality bioassay was selected due to its predictive nature for cytotoxicity and bioactivity, cost-effectiveness, ease of use, and sensitivity to toxins. Its established protocol, ethical considerations, and ability to serve as an initial screening tool further justified its relevance to our research on Irvingia gabonensis seed extracts.
This in vivo lethality test has been successfully used as a preliminary study of cytotoxic and antitumor agents.
2. Objectives
This study aimed to investigate the bioactivity and cytotoxicity of the ethanol extract and ethyl acetate derivative of Irvingia gabonensis seed, focusing on their effects on brine shrimp.
3. Methods
3.1. List of Apparatus
Rotary evaporator, vacuum oven, vacuum liquid chromatography (VLC) setup, test tubes, and glass capillary tube.
3.2. Drugs/Chemicals
Cyclophosphamide (sourced from Sigma-Aldrich), pure ethanol (sourced from Sigma-Aldrich), dimethyl sulfoxide (DMSO) (sourced from Sigma-Aldrich), sea salt (sodium chloride), 1N NaOH (sodium hydroxide), and brine shrimp eggs.
3.3. Preparation of Sample
In this research, fresh fruits and leaves of Irvingia gabonensis were gathered from Ilora in the Afijio Local Government Area of Oyo State, Nigeria. The plant specimens were authenticated by a taxonomist, Mr. Ademoriyo, and a voucher specimen (IFE-17976) was archived in the herbarium for future reference. The seeds were processed by grinding and drying the peeled fruits, which were then soaked in pure ethanol for 72 hours with periodic stirring. The resultant extract was filtered and concentrated using a rotary evaporator and a vacuum oven. Through fractionation of the concentrated crude ethanol extract with solvents of increasing polarity (n-hexane, ethyl acetate, and ethanol) in a VLC setup, three fractions were produced. These fractions were further concentrated and stored at low temperatures for later analysis. The crude ethanol extract and ethyl acetate fraction were selected for bioactivity testing because they exhibited a higher yield of phytochemicals compared to the other fractions.
Samples of the crude extract and the ethyl acetate derivative were prepared at a stock concentration of 1 mg/mL (1000 µg/mL). To achieve a stock concentration of 1000 µg/mL, 3 mg of the sample was dissolved in 3 mL of 1% DMSO, from which six graded concentrations were prepared for the study's purposes.
3.4. Brine Shrimp Lethality Bioassay
The brine shrimp lethality bioassay was conducted to assess the cytotoxicity of the extracts. Brine shrimps (Artemia salina) were hatched from eggs in a container filled with simulated sterile artificial seawater, also known as a brine solution. This solution was prepared by dissolving 38 g of sea salt (sodium chloride) in 1000 mL of distilled water and adjusting the pH to 8.5 using 1 N NaOH under constant aeration for 48 hours. The actively moving shrimps were then collected for the assay (2). Approximately 4.5 mL of the brine solution was placed into each test tube. Appropriate dilutions of the test substances (extracts) were prepared based on the required concentrations. To these test tubes, 0.5 mL of the diluted test solution was added. Ten active shrimps were introduced into each test tube using a glass capillary tube. After 24 hours, the surviving shrimps were counted, and the lethal concentration, LC50, was determined.
3.5. Calculation
The mortality endpoint for this bioassay is defined as the lack of controlled forward motion observed over a period of 30 seconds. The lethality percentage for the nauplii at each concentration and for the control was calculated by counting the number of deceased and living nauplii in each tube and determining the percentage of deaths (4, 5).
The observation period of 30 seconds is applied cumulatively to all nauplii within a specific test tube. This means that during the 30-second observation period, the absence of regulated forward movement is collectively assessed for all nauplii present in the tube, rather than assessing each nauplius individually.
3.6. Toxicity Testing Criteria
The toxicity of herbal extracts, expressed as LC50 values, is commonly evaluated by comparing them to Meyer’s or Clarkson’s toxicity indexes. According to Meyer’s toxicity index, extracts are deemed toxic if the LC50 is less than 1000 μg/mL, while those with an LC50 greater than 1000 μg/mL are considered non-toxic (6). Clarkson’s toxicity criterion for assessing the toxicity of plant extracts categorizes them as follows: Non-toxic for LC50 values above 1000 μg/mL, low toxic for LC50 values between 500 and 1000 μg/mL, medium toxic for LC50 values between 100 and 500 μg/mL, and highly toxic for LC50 values between 0 and 100 μg/mL (7).
The LC50 value, which indicates the concentration at which 50% of the brine shrimp population is affected, was determined using a standard method. Various concentrations of Irvingia gabonensis seed extracts and their ethyl acetate derivatives were prepared, typically spanning from low to high concentrations.
Subsequently, the prepared extract concentrations were added to separate test tubes containing a specified volume of brine solution. A fixed number of active brine shrimp nauplii were then introduced into each test tube, ensuring the even distribution of the test substance within the solution.
The test tubes were incubated under controlled conditions, usually at a set temperature and light cycle, for a common duration of 24 hours. During this period, the survival rate of the brine shrimp nauplii was periodically monitored.
Following the incubation, the test tubes were examined, and the counts of surviving and deceased brine shrimp nauplii in each tube were recorded. The mortality rate, often determined by the absence of controlled forward movement during a specified observation time, was utilized to calculate the lethality percentage for each concentration.
A dose-response curve was then plotted using the collected mortality data across various concentrations, from which the LC50 value was estimated via statistical methods or interpolation techniques. This estimation corresponds to the concentration causing 50% mortality.
Experiments were performed in duplicates, and statistical analysis was conducted using GraphPad Prism 5. Results are presented as means.
4. Results
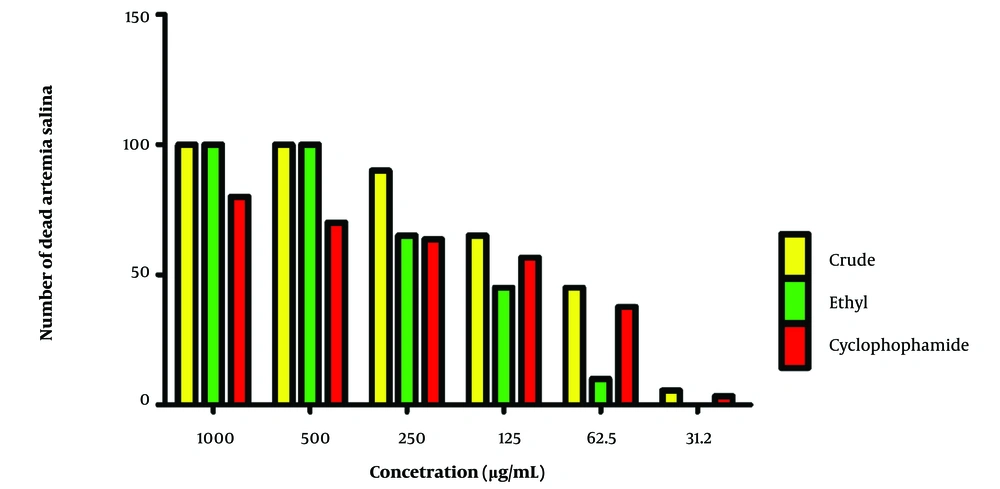
The results indicated that at the highest tested concentration of 1000 µg/mL, both the crude and ethyl acetate samples exhibited 100% cell death, showing max significant cytotoxicity at this concentration. In contrast, cyclophosphamide showed a decrease in cell death, with 80.0% of the brine shrimp dead at the same concentration. As the concentration of the samples decreased, a dose-dependent cytotoxic effect became evident. For instance, at 500 µg/mL, the cyclophosphamide sample's cell death dropped to 70.0%, while the crude and ethyl acetate samples continued to maintain 100% cell mortality. At lower concentrations of 250 µg/mL and 125 µg/mL, the cytotoxic effects of both the crude and ethyl acetate samples became decreased. The cyclophosphamide sample also showed a dose-dependent reduction in cell viability, confirming its effectiveness as a cytotoxic agent.
However, at the lowest tested concentrations, of 62.5 µg/mL and 31.2 µg/mL, the crude sample demonstrated significant cytotoxicity, with cell mortality dropping to 45.00% and 5.50%, respectively. The ethyl acetate sample exhibited lesser cytotoxicity, with cell mortality reduced to 10.00% and 0.00% at these concentrations, respectively. Cyclophosphamide, known for its cytotoxic properties, also displayed pronounced cytotoxicity at these lower concentrations, with cell mortality at 36.67% and 3.33% at 62.5 µg/mL and 31.2 µg/mL, respectively (Figure 1).
Figure 1 depicts the lethality percentage of nauplii at each concentration of the tested extracts and the control group. By varying the concentrations of the extracts, their impact on the survival of brine shrimp nauplii was monitored over 24 hours. The data provided offer insights into the cytotoxic effects of the extracts and suggest their potential bioactivity.
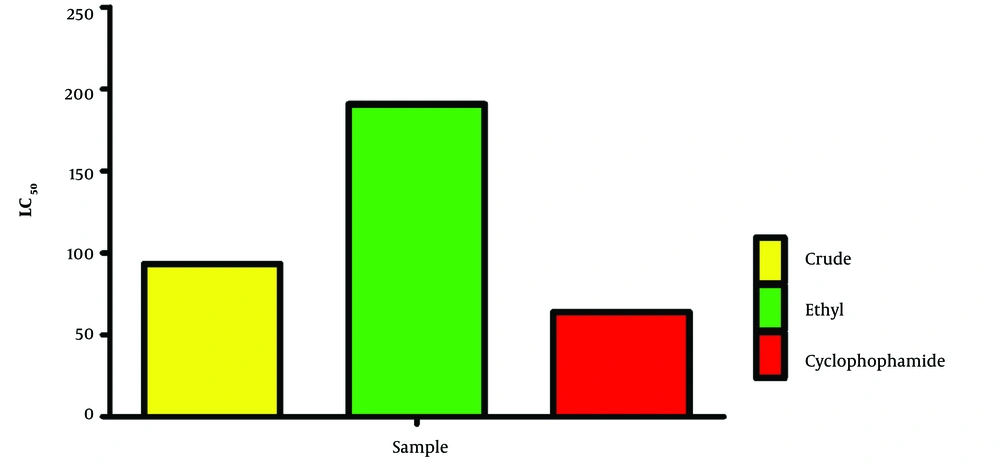
Furthermore, this study determined that the LC50 values, which denote the concentration at which 50% of the brine shrimp population was affected, stood at 93.22 ± 0.03 μg/mL for the ethanol extract and 190.80 ± 0.03 μg/mL for the ethyl acetate derivative (Figure 2). These LC50 values illustrate the concentration required to affect 50% of the brine shrimp population by each tested substance, offering a perspective on the relative cytotoxicity of the extracts in comparison to the standard drug.
The results indicate moderate cytotoxicity for both extracts, with the ethanol derivative exhibiting higher cytotoxicity than the ethyl acetate derivative. This suggests that the extraction process plays a role in the bioactivity of the compounds.
Interestingly, the LC50 values for both derivatives were found to be comparable to those of cyclophosphamide, a widely recognized anticancer drug. The observed differences in cytotoxicity between the two derivatives might stem from their distinct chemical compositions. Gas vacuum liquid chromatography analysis would likely uncover various bioactive compounds in each extract, which might work together or counteract each other, influencing the cytotoxicity observed in the brine shrimp model.
5. Discussion
The results demonstrated that both the ethanol extract and the ethyl acetate derivative of Irvingia gabonensis seed exhibit moderate cytotoxicity against brine shrimp, with LC50 values recorded at 93.22 ± 0.03 μg/mL and 190.80 ± 0.03 μg/mL, respectively. Brine shrimp (Artemia spp.) are widely utilized for preliminary screening of bioactivity and cytotoxicity due to their sensitivity to toxic substances (8). The bioactivity and cytotoxicity displayed by the seed extracts of Irvingia gabonensis against brine shrimp underscore their potential for development into natural products. The ongoing search for novel cytotoxic agents from natural sources remains critically important in drug discovery, especially with the rising challenge of drug resistance and the need for new treatment options.
A previous study by the author revealed that chemical analysis of the ethanol extract identified several bioactive compounds, such as flavonoids, terpenoids, and phenolic compounds. These compounds are known for their diverse biological activities, including antioxidant, antimicrobial, and anticancer properties (9). The presence of these bioactive compounds in the Irvingia gabonensis seed extracts may underlie their cytotoxic effects observed in brine shrimp. Identifying these compounds provides a molecular basis for the cytotoxic effects seen, aligning with previous research that has highlighted Irvingia gabonensis's therapeutic potential. These findings endorse further exploration into the cytotoxic properties of Irvingia gabonensis (10, 11).
Flavonoids are celebrated for their robust antioxidant activity, which plays a pivotal role in their anticancer properties by neutralizing free radicals, mitigating oxidative stress, and fostering cell survival (12). These compounds excel in scavenging free radicals and curtailing oxidative stress, thus safeguarding cells from DNA damage and enhancing cell survival (13-16). Conversely, terpenoids have shown a broad spectrum of biological activities, including cytotoxicity against cancer cells through various mechanisms, such as hindering cell cycle progression, prompting apoptosis, and disturbing cellular signaling pathways (17, 18). The structural variability of terpenoids enables them to engage with multiple molecular targets, offering diverse mechanisms of action against cancer cells (19). Similarly, phenolic compounds, including tannins and phenolic acids, have demonstrated cytotoxic and anticancer activities by triggering apoptosis, impeding cell proliferation, and interfering with cellular signaling pathways (18-20). The observed higher cytotoxicity in the ethyl acetate derivative compared to the ethanol extract may be attributed to the differential presence of bioactive compounds, underscoring the significance of fractionation methods in pinpointing potent cytotoxic agents. Delving into the chemical composition responsible for this discrepancy can provide crucial insights for future drug development targeting cancer therapy.
The moderate cytotoxicity noted in this study indicates that Irvingia gabonensis seed extracts likely harbor bioactive compounds with potential anticancer properties (21). Future research should not only assess cytotoxicity but also aim to unravel the mechanism of action of the bioactive compounds found in Irvingia gabonensis seed extracts (22). Uncovering the molecular targets and pathways they influence will shed light on valuable opportunities for drug discovery and development. Moreover, investigating the selectivity of these compounds for cancer cells over normal cells is essential to determine their viability as therapeutic agents.
While this study yields encouraging results concerning the cytotoxicity of Irvingia gabonensis seed extracts, it is crucial to acknowledge that the brine shrimp lethality assay, being a simplistic model system, may not comprehensively represent the extracts' cytotoxic effects on mammalian cell lines or in animal models. Additionally, it is important to recognize the limitations and challenges inherent in research involving natural products. These products are complex mixtures of compounds, and their bioactivity may vary due to factors like extraction methods, geographical location, and seasonal changes. Moreover, the cytotoxicity observed in brine shrimp does not automatically imply effectiveness in humans. Consequently, detailed preclinical and clinical studies in more relevant models are essential to ascertain the safety, efficacy, and possible side effects of these extracts before considering them for therapeutic applications.
5.1. Conclusions
In summary, this study highlights the moderate cytotoxicity exhibited by the ethanol extract and its ethyl acetate derivative of Irvingia gabonensis seeds against brine shrimp. The detection of bioactive compounds, such as flavonoids, terpenoids, and phenolic compounds, underlines a molecular foundation for their cytotoxic effects. These preliminary findings advocate for further exploration of Irvingia gabonensis seeds as a source of natural products with cytotoxic attributes. Nonetheless, further research is necessary to confirm these cytotoxic effects in mammalian cell lines and animal models, uncover the mechanisms of action, and evaluate their therapeutic potential. The pursuit of novel anticancer agents from natural sources is a significant aspect of drug discovery, and Irvingia gabonensis seed extracts exhibit potential in this domain.