1. Background
The aging community is growing worldwide. Population growth has increased geometrically, rising by 5.2% from 2009 to 2015, resulting in 40,000 middle-aged and 250,000 elderly individuals (1). Additionally, the prevalence of obesity in these age categories increased from 16% to 18%, with women at a higher risk than men across all continents, both in developed and developing countries (2). Alarmingly, a recent study revealed a decline in public awareness and health-seeking behaviors regarding obesity from 2004 to 2021 (3). If this trend continues, a majority of the world's adult population will be either overweight or obese by 2030 (4). However, these figures, derived from BMI-based estimates, are not highly sensitive tests for determining obesity (5), and the true prevalence is likely much higher than what these tests regularly report (6). Given the significant underestimates of obesity prevalence and its association with multiple comorbidities (7), as well as being a gateway to many non-communicable diseases (8) leading to a high risk of hospitalizations and intubation (9), different preventive and therapeutic measures to assist the daily living activities of the elderly, most directly impacted by obesity, would be beneficial. This need is more pressing than ever, especially in light of the world’s new COVID-19-related challenges and other global crises predicted in the coming years (10). Furthermore, the role of obesity as a risk factor has been emphasized by data collected across all ethnicities during the current SARS-CoV-2 pandemic (11, 12).
The impact of obesity on falls has not been thoroughly examined. Rosenblatt and Madigan and Choi et al. identified a strong positive correlation between fat mass index and trip-induced fall risk among older adults (13, 14). This finding is recently supported by Thiamwong et al., who demonstrated that body fat mass (BFM), percent body fat (PBF), and Body Mass Index (BMI) are correlated with fear of falling (FoF) and balance impairment (15). Furthermore, a distinct pattern of force application during walking has been observed between obese individuals and weight-matched control groups (14), suggesting that obesity may contribute to fall risk, although it is not the sole factor (16). Other independent factors such as disability, history of falls, hand grip strength (HGS), number of medications used, and pain have also been shown to influence FoF (17). The complexity of these factors, combined with the use of different stability measures, may explain why some studies have found no effect of obesity on gait stability in this population (18). Greater muscle weakness in the lower limbs and 34% balance disorders are common in older adults, which may be exacerbated by a variety of biological and social factors, including physical disability, chronic illnesses, incontinence, inactivity, and obesity (19). Regarding the role of gender, both younger women under 50 years of age (20, 21) and older women aged 65 to 75 years (22) exhibit lower stability limits, making them more susceptible to falls. However, irrespective of gender, obesity alone leads to altered postural control, associated with increased activity of plantar flexion. This underscores the importance of focusing on proprioception and neuromuscular control in fall prevention rehabilitation protocols (23). This is why individuals with obesity are often twice as likely to fall as those who are not obese (24).
In recent years, there has been growing interest in using controlled whole-body vibration (CWBV) training not only as an alternative rehabilitation method for fall prevention but also across various fields exploring a wide range of demand-based applications. These include improving overall fatigue (25) and athletic performance (26), and adopting recovery-oriented approaches for the rehabilitation of neurological (27), musculoskeletal (28), and metabolic conditions by inducing biological responses that enhance functional parameters in these patients (29). However, despite promising results regarding the efficacy of WBV or CWBV combined with exercise in improving balance and proprioception, a recent systematic review indicated that no definitive conclusions could be drawn due to the heterogeneity of articles, duration of exposure, parameters characterizing mechanical vibration, and the lack of clinical trials exploring different frequencies and amplitudes (30). Therefore, identifying these contributing factors through various assessment models should be among the first steps toward developing appropriate intervention strategies to reduce the fall rate.
The neuromuscular adaptations acquired through CWBV appear to be an important mechanism for improving basic living skills in older adults by enhancing proprioceptive sense. As a complementary therapy, this technique, alongside unstable shoes, could be proposed as a beneficial intervention with relatively long-term effects on balance measures in older people (31). A recent study concluded that this model may effectively improve neuromuscular performance and balance in healthy individuals (28), as well as in patients using CWBV for neurological recovery. Chang et al. reported altered neuromuscular control in patients with Parkinson’s disease through a pilot analysis (27), Ruhde and Hulla observed improvements in physical function in individuals with cerebral palsy (CP) (32), Li et al. described this technique as a passive and safe clinical intervention as an alternative treatment for individuals with motor impairments or poor balance function (33), and Guadarrama-Molina et al. recently proposed this therapy as a co-adjuvant to conventional therapy for improving functional balance status (34). However, some studies report the opposite, with no motor/balance symptom-reducing effect of CWBV (35), and the reasons behind this should not be underestimated. Implementation also contributes to these discrepancies, such as the different stances on a force plate—semi-squat position or deep-squat position (36)—and performing dynamic exercises, such as squats or leg lunges, while on the plate. The frequencies of vibrations used in studies ranged from 10 to 50 Hz, with amplitudes from 1 to 5 mm, and some studies progressively increased vibration frequencies and amplitudes from session to session; however, using a 20 Hz frequency and 2 mm amplitude applied in 30 to 60-second intervals (36) and a 30 or 60-second interval (37) has been reported to be most beneficial in improving balance, supported by an earlier study that reported improved functional gait efficiency, dynamic balance, functional strength of lower body muscles, and exercise tolerance in women over 60 at increased risk of falling (38).
The complexity and multifactorial nature of falls, along with challenges in measuring proprioception in older adults with obesity, have led to conflicting findings and hindered our understanding of the neurophysiological mechanisms of vibration. This understanding is key to prescribing a safe and effective CWBV protocol aimed at reducing the aging effects on the musculoskeletal structures of obese individuals prone to falling. To date, studies investigating the health-enhancing effects of CWBV—either alone or in conjunction with other therapies—specifically in obese individuals at risk of falling, have not reached a consensus to ascertain its potential impact on balance parameters and home-related falls to aid these individuals in maintaining the physical activity they need. Thus, this should also be considered in addition to addressing the obesity-associated metabolic parameters that conventional therapeutic protocols have focused on.
2. Objectives
To advance our understanding, as prompted by other researchers' recent endeavors, this pilot study aims to investigate the effects of CWBV on balance parameters and fall risk among obese adult women. The hypotheses are as follows: First, the therapeutic effect of CWBV on balance parameters and FoF is significantly different from that of conventional therapies routinely used in older adult women with obesity. This is because CWBV offers a more versatile and patient-centered rehabilitation protocol, meeting users' diverse physical fitness needs and specific requirements through adjustable vibration setups and tailored degrees of muscle activation. Secondly, CWBV training may provide a quicker additional modality to enhance the active lifestyles of this population by improving stability and addressing personal concerns about falling.
3. Methods
3.1. Participants
Fifteen adult women with obesity, who are employees at a university's biomechanical research center and aged over 60 (68.55 ± 4.86) with a BMI of 30.57 ± 2.97, were voluntarily recruited and participated in this study. The study used a one-group pretest-posttest design (Figure 1 and Table 1). The exercise and measurement protocols were conducted in accordance with the Declaration of Helsinki-Tokyo and approved by the local ethics committee.
After obtaining the necessary permissions for research, candidates completed a medical history form and a consent letter. BMI, a reliable indicator of body fatness with high sensitivity and specificity for individuals over 60 (29), was used to assess obesity and to include only those with a BMI over 30. Our inclusion criteria were as follows: (a) Having normal vision, hearing, and dexterity; (b) no personal history of cancer, diabetes, mental or neurological disorders, or other significant diseases that could bias the results or contraindicate vibration exercise; (c) not having taken any medications that could affect balance or movement smoothness (either increasing or reducing movement speed, whether voluntary or involuntary) in the last six months; and (d) having physical and cognitive abilities confirmed through medical records and being interested in participating in the intervention program. Participants who did not meet these criteria were excluded from the study. A table of random numbers was used to create our final sample. Initially, each participant was assigned a number between 1 and 16. Then, a team member blindfolded herself and randomly pointed to a spot on the chart containing three-digit numbers, selecting the first two digits one time and the first digit another time to match one of our subjects until we identified our final fifteen participants for the intervention.
3.2. Intervention
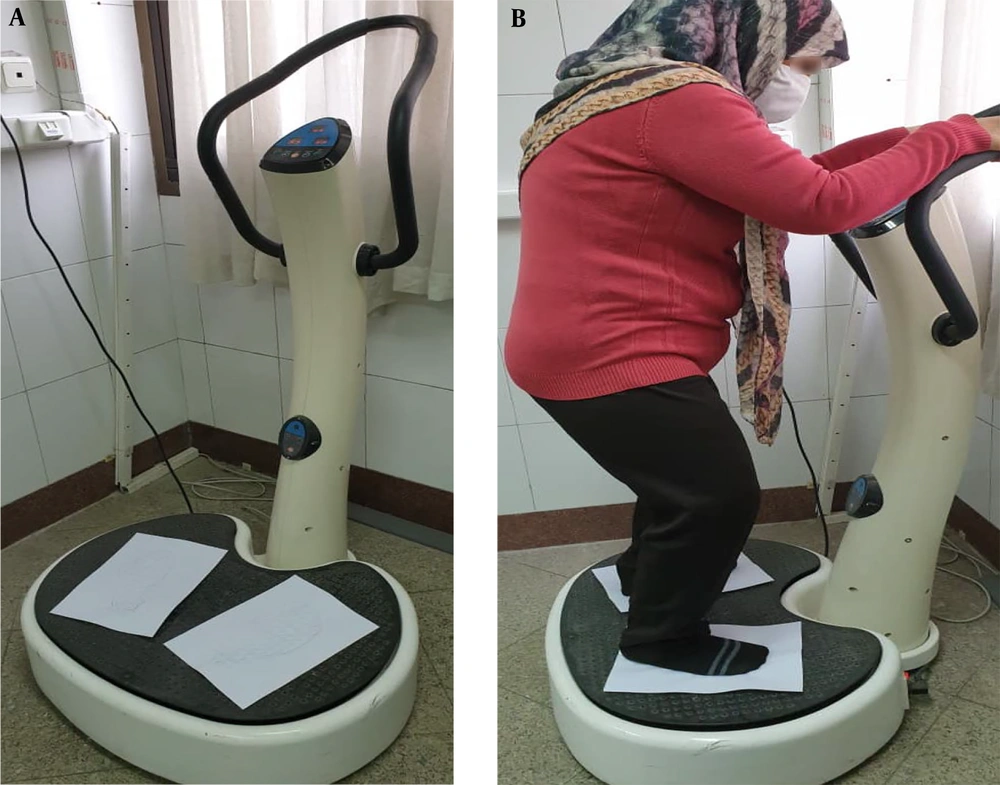
After identifying participants who met the inclusion criteria, they first received a detailed explanation of how to use the device effectively while adhering to all safety precautions during a session of familiarization trials. Subsequently, all participants were assigned to CWBV training three times weekly for six weeks under the supervision of an experienced physiotherapist and exercise physiologist. Each session lasted on average 10 minutes, with participants standing with knees flexed at 20 degrees and their trunk in an upright vertical position on the marked footprint over the vibration platform (Extream1000; AMH International Co., Ltd., Incheon, Republic of Korea). They underwent five cycles of one-minute exposure to the vibration training protocol with a one-minute rest interval between cycles and a vibration frequency set at a maximum of 30 Hz (Figure 2). The vibration intensity was progressively increased by adjusting the frequency up to 30 Hz and the amplitude up to 1 - 2 mm (39, 40).
3.3. Measurement
Recent studies have identified practical functional balance measures with the highest levels of ability to indicate the risk of falls among older adults, boasting a sensitivity of 92.68%, specificity of 84.09%, and an AUC of 0.931 (95% CI = 0.860 to 1.000) (41). These tests, suggested for roughly gauging individual functional levels, are simple, feasible, and practical tools that can be administered to larger populations and conducted almost anywhere. Therefore, static and dynamic balance parameters were selected for evaluation before starting CWBV training and again six weeks afterward, using the Zebris FDM system (ZEBRIS Medical GmbH, Germany)—a portable force platform that provides access to spatio-temporal gait parameters with high accuracy. The reliability of this system has been reported as acceptable to excellent in balance assessments by Van Alsenoy et al. (42).
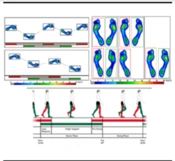
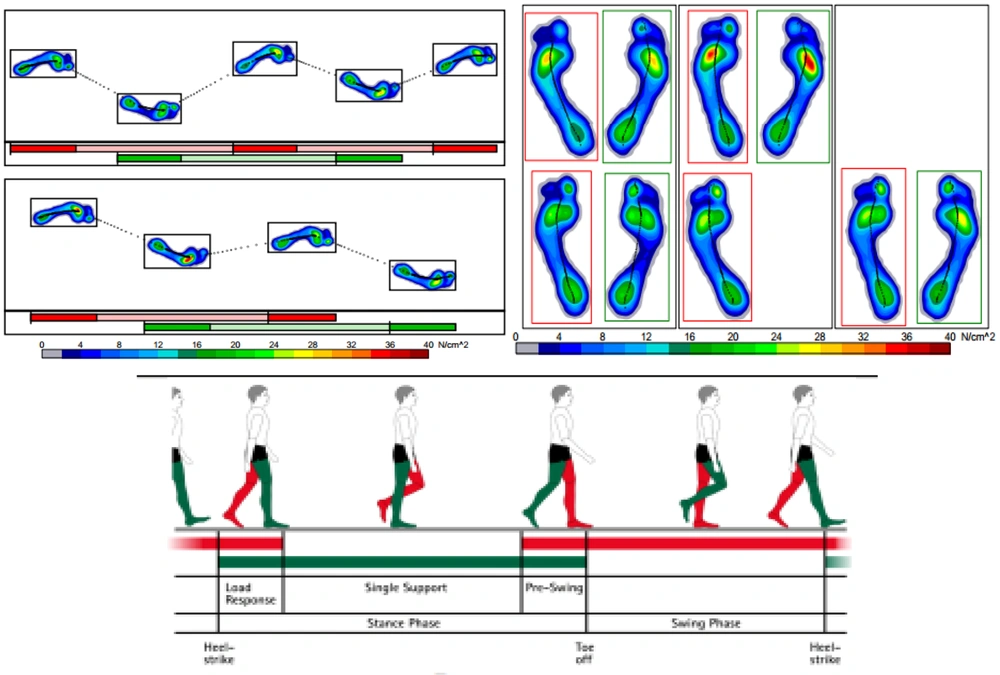
Static balance was assessed over a 20-second period with subjects standing barefoot on the platform, feet shoulder-width apart. Dynamic balance was evaluated through a walking test where subjects walked barefoot on a 2-meter platform at their comfortable and safe speed (Figure 3).
The analysis of the force's application point between the feet and the ground was based on Romberg's test, assessing static balance during free-standing on both lower limbs with eyes alternately closed and open. Ellipse sway area (ESA) (COP displacement) and path length (the distance the COP covers) were selected to determine static balance. For dynamic balance, step width (the distance between the line of progression of the left foot and the line of progression of the right foot measured in cm), total double support (duration of the gait cycle phase when both lower limbs are in contact with the treadmill belt, recorded and expressed in percent), stride length (data from overground walking gait used to calculate stride length), stride time (duration of foot displacement between two consecutive steps on the same side, recorded and expressed in ms), cadence (number of steps per minute—sum of both feet in this study), and velocity (time taken by each subject to traverse the entire distance, measured in m/s), were all monitored according to their standard definitions and norms (43) to assess dynamic stability. The mean values of three repetitive trials for each pre and post-test were taken as the test outcomes. All data were then recorded and stored on a laptop. Test-retest reliability and validity of these center of pressure-based parameters were explored and found to be acceptable for assessing balance in older adults, both standing with eyes opened or closed, and also in seated positions (ICC = 0.99) (44).
To assess FoF, the Fall Efficacy Scale-International (FES-I) was used. The country-specific standardized version of FES-I has been found acceptable to assess FoF among older adults (45), as well as the international version (46, 47). In this test, FoF is operationalized as the level of concern about falling over 16 different physical activities. It has a four-point scale ranging from 1 (not concerned) to 4 (very concerned), resulting in a total sum-score between 16 and 64— the higher each parameter score, the higher the levels of concern/FoF.
3.4. Statistical Analysis
We first estimated the number of participants using G*Power (Version 3.1.9.4.) (48), required for a significant group by time (pre, post) interaction. This test revealed that enrolling 15 patients for the intervention phase would produce a medium effect of 0.5 (alpha: 0.05, power: 0.82). The results of the Kolmogorov–Smirnov test did not confirm normally distributed data in all variables (P-value ≤ 0.05), and the Wilcoxon signed-rank test, as a non-parametric paired t-test, was used accordingly to examine pre-post-treatment changes. Meanwhile, statistical analysis was carried out using SPSS (IBM, USA, version 17.0) with a 95% confidence interval.
4. Results
According to Table 2, a significant difference was observed in all parameters of both static and dynamic balance, including Ellipse Area (P = 0.008, ES: 1.82) and path length (P = 0.013, ES: 2.66), step width (P = 0.03, ES: 0.78), total double support (P = 0.021, ES: 0.73), stride length (P = 0.022, ES: 0.76), stride time (P = 0.003, ES: 1.18), cadence (P = 0.003, ES: 1.06), and velocity (P < 0.001, ES: 1.06), except for the variability of velocity (P = 0.9) in the pretest-posttest score comparison. The test result of the FES-I questionnaire (P = 0.56) also found no statistically significant difference in FoF following the intervention. Meanwhile, the formula Meanpost – Meanpre / Sdpre was applied for this one-group pre-post design to assess the effect size of each parameter. According to the study of Mordkoff, the test of all variables is interpreted as having a large effect size—0.01, 0.06, and 0.14 indicate small effect, medium effect, and large effect, respectively (49, 50).
| Stability Parameters | Condition | P-Value b | |
|---|---|---|---|
| Pre-intervention | Post-intervention | ||
| Static balance | |||
| Ellipse Area1 (mm2) | 99.04 (3.906) | 91.90 (3.812) | 0.008 |
| Path length2 (mm) | 147.92 (2.887) | 140.24 (3.109) | 0.013 |
| Dynamic balance | |||
| Step width | 13.00 (2.8) | 10.81 (2.48) | 0.03 |
| Total Double Support | 36 (3.8) | 33.2 (4.7) | 0.021 |
| Stride length | 88.8 (10.4) | 95.18 (11.01) | 0.022 |
| Stride time | 1.28 (0.11) | 1.15 (0.08) | 0.003 |
| Cadence | 47.09 (4.78) | 52.18 (3.97) | 0.003 |
| Velocity | 2.56 (0.48) | 3.07 (0.52) | < 0.001 |
| Variability of Velocity | 6.54 (1.50) | 6.54 (2.16) | 0.9 |
| FES-I3 score (range 1 to 64) | 25.55 (4.19) | 24.3 (5.1) | 0.56 |
Descriptive Data and Wilcoxon Signed-Rank Test for Parameters of Static and Dynamic Balance, and FES-I Test Before and After Treatment a
5. Discussion
To begin with an overview of the findings, the improvement of static and dynamic balance reported among adult women with obesity following our CWBV training is clearly the main scientific discovery of the present study. However, this technique did not support our pre-study hypothesis regarding the efficiency of CWBV intervention on FoF in obese fallers.
Whole-body vibration has recently been found to be a potential tool in the rehabilitation of the elderly and patients with chronic diseases. However, few studies have been performed concerning whole-body vibration in these individuals who exhibit poor balance performing ability and to investigate its potential impact on proprioception to clarify the connection between proprioceptive function and improved motor function. Among all the well-documented obesity comorbidities, balance impairment has always been one of the most ignored risk factors that had to be addressed as equal to metabolic comorbidities in healthcare and rehabilitation programs for older adults with obesity. Although metabolic healthy obesity (MHO) shows reduced cardiometabolic risks and MHO individuals are closer to normal than those with unhealthy phenotypes (51), it could not yet be considered a safe condition (52), and a modest weight loss (5 - 10% of body weight) has been proven to alleviate their cardiovascular risk (53), recently supported on a broader scale by Haase et al. (54). Increasing ambulatory activity might also be one of the other clues to solving the increasing prevalence of obesity and its associated comorbidities (55). Therefore, the present study is one of the first studies aimed to provide evidence in support of an additional effect of CWBV training on stability parameters while verifying the efficiency of its controlled exposure on the risk of fall and on the active life of our older adult women with obesity (20).
What this study found may coincide with the results of studies that reveal improvements in balance and postural control following CWBV training. Onal et al. reported significant improvements in static and dynamic balance among stroke patients but not obese individuals (56). Melo et al. , through a recent systematic review, found positive results when vibration—with frequency values of 30 to 35 Hz as set in our study’s protocol—was used alone or in association with other types of exercises in older adults with obesity (57). Maghbouli et al. reported that CWBV training using a vibration frequency higher than 100 Hz may improve muscle strength and open-eye mediolateral postural control, recommending this technique as a promising approach for decreasing fall risk (58). Additionally, several recent studies found this modality to be an effective stimulus to improve neuromuscular performance and balance in healthy individuals (26), as well as in elderly individuals with neurological disorders (27, 32).
Looking at these previous studies, it has been shown that most of the physical activity-induced health benefits have been acquired from the simplest, most fun, and entertaining programs, which provide a sufficient training stimulus that older adults are able to comply with and complete eagerly due to age-related physical debilities. This is partly why CWBV training seems capable of delivering the same benefits seen with conventional exercise programs, but in a simpler format that allows older adults to be mentally and physically engaged on a routine basis. However, a few studies that failed to show any positive effect of this technique (35, 59) highlight the importance of setting up an optimal amplitude and frequency of the vibration, coupled with an optimal level of muscle activity by which the vibration stimulation could be superimposed.
For instance, the study conducted by Miller et al. shows that exposure to continuous whole-body vibration may reach its peak application with the participant on the platform, allowing fatigue to set in, speculating greater change post-CWBV possibly due to the balance of potentiation and fatigue, which is missed in our vibration protocol and other intermittent whole-body vibrations potentiate performance without fatigue accumulation (59). In another study, fixed-frequency and amplitude vibration training did not result in any meaningful changes in any of the balance variables (60), where the exact mechanism behind this failure compared to stability improvements concluded by the protocol designed an individualized vibration frequency and amplitude according to their EMG data, may be attributed to the provision of stronger stimulation to skin receptors, muscle spindles, and the vestibular system. Other than that, most of the studies have focused on the leg extensors, neglecting plantar flexors, which have been shown to be up to five times more effective in increasing electromyographic activity with vibration. All these factors may explain in part why our CWBV protocol, despite the balance improvements, was not sufficient to impact the fall efficacy scale, which reflects real-life functional limitation-related concerns.
In the case of older adults with obesity specifically, FoF and its relationship with activity have not been sufficiently investigated. Rosic et al. appear to be the most recent study suggesting that FoF is associated with low levels of physical activity (20). However, no links have been found between FoF and obesity (61), except for some conflicting findings demonstrated contrariwise (62-64). Perhaps a new somato-sensory stimulation (SSS) type of exercise would improve these fall-related psychological concerns through triggering brain plasticity (65), or even historical derivatives of Chinese healing practices based on mind–body interaction protocols, as recently claimed by Nissim et al. (66), would be a better option to enhance cerebellar activation, which in turn might improve balance. This is because, compared to voluntary muscle control during conventional exercise training, these techniques rely more on contractions induced by passive stretch reflexes, leading to facilitating the excitability of the spinal reflex. After reaching this level of physiological and neuro-muscular adaptations (67), our older adults with obesity would end up with a more adaptable body, making its control easier to perform smoother movements (68)—as compensation for all possible age-related physical debilities and inactivities they are most likely exposed to. Therefore, despite the lack of significance of FoF, our results imply clinical relevance particularly in individuals showing contraindications toward conventional exercise therapies, considering and addressing the limitations of the present study listed as follows.
5.1. Limitations
The present study has some limitations that need to be discussed and considered thoroughly by future works. First, the sample size was small, as reasons such as not exceeding the budget and not violating our intraorganizational rules and policies (not recruiting subjects outside of the department) hindered us from enrolling a higher number of participants. Secondly, there is not yet standardization of a vibration protocol for obese individuals in the literature. Therefore, we tried to apply the most commonly used protocol with the vibration setup designed by evidence-focused databases, but not with individualized vibration that—based on others’ up-to-date findings—may present stronger stimulation to the body than the fixed–frequency and amplitude vibration training being used in the present study. Thirdly, daily activity, work, smoking, physical activity, and daily energy intake could all be disturbing variables which were not controlled and may have affected the present findings. Fourthly, this study was limited to the short-term effect of CWBV training for obese individuals and the findings are not generalizable beyond this scope; thus, the long-lasting effects of this technique need to be investigated. Fifthly, this study was also restricted to older adult women as they have been shown to have higher levels of FoF in the elderly than men. Lastly, this is an exploratory study conducted without a control group, which reflects back to our criteria and the number of patients that fit it when undertaking their routine treatment in the physiotherapy department.
5.2. Conclusions
We consider CWBV training to be an effective method for improving static and dynamic balance parameters in older adult women. However, further research is warranted to explore different dosages of vibration protocols or to incorporate more challenging/specialized proprioceptive exercises into CWBV. This could help target the improvement of FoF in those who are unable to participate in other forms of conventional exercise programs for rehabilitation. Additionally, it is important for further studies, in addition to addressing the aforementioned limitations of the present study, to explore the neurophysiological mechanisms involved in muscle activation with individualized vibration. This will enable the prescription of safe and more effective CWBV programs, addressing possible age-related physical debilities and inactivities that these individuals are likely to suffer from.