1. Background
Pregnancy is a transitional period marked by significant physical and emotional changes. These changes can sometimes lead to issues, including nausea and vomiting, gestational diabetes, urinary infections, blood pressure fluctuations, anemia, sudden pain under the ribs, edema, and increased vaginal discharge (1).
Urinary tract infection (UTI) is the most common bacterial infection globally. Its prevalence increases during pregnancy due to various physiological changes in women (2). Urinary stasis and bladder reflux, primarily caused by hormonal and mechanical changes, are among the main causes of UTIs. Additionally, the urethra's shortness and the challenge of maintaining hygiene due to abdominal expansion make UTIs the most frequent bacterial infection in pregnancy (3).
This condition is linked with several adverse maternal and neonatal outcomes, such as low birth weight, premature birth, stillbirth, preeclampsia, maternal anemia and sepsis, and amnionitis (4, 5). Risk factors include a previous history of UTIs, pre-existing diabetes, higher parity, low socio-economic status, immunosuppression, smoking, maternal age, and delayed prenatal care. Studies have also indicated a higher prevalence among individuals with a history of UTIs (6).
Anemia is another prevalent issue during pregnancy. The mother's blood volume increases by 30 - 50%, but the plasma volume increases more than the red blood cell volume, leading to diluted blood and a decrease in blood hemoglobin levels. This results in physiological anemia during pregnancy (7). While mild iron deficiency anemia may not cause significant fetal complications, severe anemia (hemoglobin below 7) can lead to serious adverse outcomes, including spontaneous abortion, preterm birth, low birth weight, and fetal death (8).
2. Objectives
Due to the maternal and fetal complications arising from pregnancy disorders, it is crucial to identify mothers facing these challenges. Given the lack of research in Khash city, this study was initiated to determine the prevalence of pregnancy disorders among women visiting health centers in Khash city.
3. Methods
This cross-sectional study was conducted on pregnant women attending health centers in Khash city, southeastern Iran. The study included all pregnant women who had opened a pregnancy file at one of the comprehensive health centers, received pregnancy care and underwent routine pregnancy tests in 2022. The selection was made using the census method. Pregnant women with a history of anemia prior to pregnancy, urinary tract abnormalities, incomplete health center records, those who used psychotherapeutic drugs, or had their pregnancy terminated before 24 weeks were excluded from the study. Additionally, the pregnancy week did not influence study participation. In total, 700 pregnant women were included in this study. Data collection was facilitated through a self-administered checklist covering sociodemographic and clinical factors such as age, education level, employment status, husband's occupation, income level, BMI, smoking habits, number of deliveries, number of medical care visits, interval between pregnancies, use of iron and folic acid supplements, diabetes, thalassemia, history of UTIs, hypertension, kidney disease, constipation, history of abortion, venereal disease research laboratory (VDRL) test results, gestational age, and the interval between pregnancies.
The data were analyzed using the SPSS software package, version 22. Categorical variables were described using frequency and percentage, while continuous variables were described using mean and standard deviation (SD). The chi-square test and multiple logistic regression analysis were employed to identify demographic and obstetric factors associated with UTI and anemia. A P-value of less than 0.05 was considered statistically significant.
4. Results
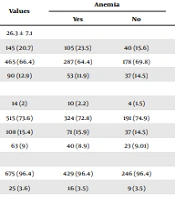
The average age of the pregnant women participating in the study was 26.3 ± 7.1 years, with most of the participants falling within the 20 - 35 age range. About 2% were illiterate, while 73% had completed secondary school education. Over 90% were housewives. Approximately 50% of the women had a normal BMI. A majority of the pregnant women (63%) did not smoke during pregnancy. More than half of their husbands were self-employed and had a low income (Table 1). Additionally, 50.4% of the pregnant women were beyond 27 weeks of gestation. Most of the women had received healthcare services fewer than 5 times (74%), 61% had an interval of less than 2 years between pregnancies, and 75% had more than 2 deliveries. About 14% had a history of abortion. Over 50% used supplemental pills during pregnancy. Regarding health conditions, 17% had diabetes, 10% had hypertension, 46% had thalassemia minor, and 0.6% had thalassemia major.
| Variables | Values | Anemia | P-Value | Urinary Tract Infection | P-Value | ||
|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | ||||
| Age | 26.3 ± 7.1 | 0.40 | 0.41 | ||||
| < 19 | 145 (20.7) | 105 (23.5) | 40 (15.6) | 77 (22.4) | 68 (19.04) | ||
| 20 - 35 | 465 (66.4) | 287 (64.4) | 178 (69.8) | 226 (65.8) | 239 (66.9) | ||
| > 35 | 90 (12.9) | 53 (11.9) | 37 (14.5) | 40 (11.6) | 50 (14) | ||
| Educational level | 0.87 | 0.04 | |||||
| Illiterate | 14 (2) | 10 (2.2) | 4 (1.5) | 4 (1.1) | 10 (2.8) | ||
| Secondary School | 515 (73.6) | 324 (72.8) | 191 (74.9) | 256 (74.6) | 259 (72.5) | ||
| High school | 108 (15.4) | 71 (15.9) | 37 (14.5) | 52 (15.1) | 56 (15.6) | ||
| College | 63 (9) | 40 (8.9) | 23 (9.01) | 31 (9.03) | 32 (8.9) | ||
| Occupation | 0.96 | 0.26 | |||||
| Housewife | 675 (96.4) | 429 (96.4) | 246 (96.4) | 328 (95.6) | 347 (97.1) | ||
| Employed | 25 (3.6) | 16 (3.5) | 9 (3.5) | 15 (4.3) | 10 (2.8) | ||
| Husband's job | 0.15 | 0.27 | |||||
| Unemployed | 92 (13.1) | 66 (14.8) | 26 (10.1) | 48 (13.9) | 44 (12.3) | ||
| Non-governmental | 403 (57.6) | 243 (54.6) | 160 (62.7) | 201 (58.6) | 202 (56.5) | ||
| Employee | 71 (10.1) | 46 (10.3) | 25 (9.8) | 38 (11.07) | 33 (9.2) | ||
| Manual worker | 134 (19.1) | 90 (20.2) | 44 (17.2) | 56 (16.3) | 78 (21.8) | ||
| Income level | 0.35 | 0.01 | |||||
| Low income | 421 (60.1) | 212 (47.6) | 209 (81.9) | 202 (58.8) | 219 (61.3) | ||
| High income | 279 (39.9) | 233 (52.3) | 46 (18.03) | 141 (41.1) | 138 (38.6) | ||
| Body mass index | 0.10 | 0.75 | |||||
| < 18.5 | 73 (10.4) | 54 (12.1) | 19 (7.4) | 32 (9.3) | 41 (11.4) | ||
| 19 - 24.9 | 336 (48) | 216 (48.5) | 120 (47.05) | 169 (49.2) | 167 (46.7) | ||
| 25 - 29.9 | 184 (26.3) | 115 (25.8) | 69 (27.05) | 88 (25.6) | 96 (26.8) | ||
| > 30 | 107 (15.3) | 60 (13.4) | 47 (18.4) | 54 (15.7) | 53 (14.8) | ||
| Smoking | 0.03 | 0.45 | |||||
| No | 261 (37.2) | 154 (34.6) | 107 (41.9) | 356 (87.5) | 280 (96.2) | ||
| Yes | 439 (62.7) | 291 (41.5) | 148 (58) | 52 (12.7) | 11 (3.7) | ||
Socio-Demographic Characteristics and Associated Risk Factors of Urinary Tract Infection Among Pregnant Women a
The prevalence of anemia among the pregnant women was 63.6% (95% confidence interval [CI]: 59.8 - 67.1). There was a statistically significant association between anemia and the number of medical care visits, smoking, the use of iron and folic acid supplements, history of abortion, and Thalassemia (P-value < 0.05). Furthermore, the results of the multiple logistic regression revealed that receiving medical care fewer than 5 times increased the risk of anemia by 20.3 times (95% CI = 6.7 - 61.4). Additionally, a history of abortion increased the risk of anemia by 2.6 times (95% CI = 1.5 - 4.4).
The prevalence of UTI among pregnant women was 49% (95% CI: 45.2 - 52.7), with the highest prevalence observed among women with a history of UTI, those who had completed secondary school, and those with lower income levels (P-value < 0.05). A significant association was also found between having syphilis and UTI (P-value = 0.05). According to the results of the multiple logistic regression analysis, having a history of UTI increased the risk of UTI by 6.9 times (95% CI = 2.5 - 18.8) (Tables 2 and 3).
| Variables | Values | Anemia | P-Value | Urinary Tract Infection | P-Value | ||
|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | ||||
| Age of pregnancy | 0.57 | 0.28 | |||||
| < 26 week | 347 (49.6) | 217 (48.7) | 130 (50.9) | 163 (47.5) | 184 (51.5) | ||
| > 26 weak | 353 (50.4) | 228 (51.2) | 125 (49.01) | 180 (52.4) | 173 (38.3) | ||
| Number of deliveries | 0.99 | 0.35 | |||||
| More than 2 time | 530 (75.7) | 337 (75.7) | 193 (75.6) | 265 (77.2) | 265 (74.2) | ||
| Less than 2 time | 170 (24.3) | 108 (24.2) | 62 (24.3) | 78 (22.7) | 92 (25.7) | ||
| Number of received medical care | 0.01 | 0.35 | |||||
| Less than 5 time | 519 (74.1) | 316 (71.01) | 203 (79.6) | 249 (72.5) | 270 (75.6) | ||
| More than 5 time | 181 (25.9) | 129 (28.9) | 52 (20.3) | 94 (27.4) | 87 (24.3) | ||
| Interval of previous pregnancy | 0.22 | 0.78 | |||||
| Less than 2 month | 427 (61) | 279 (62.6) | 148 (58.03) | 211 (61.5) | 216 (60.5) | ||
| More than 2 month | 273 (39) | 166 (37.3) | 107 (41.9) | 132 (38.4) | 141 (39.4) | ||
| Use of iron and folic acid supplements | 0.02 | 0.13 | |||||
| Yes | 657 (93.9) | 414 (93.03) | 243 (95.2) | 325 (94.7) | 332 (92.9) | ||
| No | 43 (6.1) | 31 (6.9) | 12 (4.7) | 18 (5.2) | 25 (7) | ||
| Diabetes | 0.16 | 0.64 | |||||
| Yes | 119 (17) | 69 (15.5) | 50 (19.6) | 56 (16.3) | 63 (17.6) | ||
| No | 581 (83) | 376 (84.4) | 205 (80.3) | 287 (83.6) | 294 (82.3) | ||
| Thalassemia | 0.001 | 0.10 | |||||
| Minor | 327 (46.7) | 325 (73.03) | 2 (0.7) | 156 (45.4) | 171 (47.8) | ||
| Major | 4 (0.6) | 1 (0.2) | 3 (1.1) | 0 (0) | 4 (1.1) | ||
| None | 369 (52.7) | 119 (26.7) | 250 (98.03) | 187 (54.5) | 182 (50.9) | ||
| History of UTI | 0.65 | 0.02 | |||||
| Yes | 285 (40.7) | 154 (34.6) | 131 (51.3) | 198 (57.7) | 87 (24.3) | ||
| No | 415 (59.3) | 291 (65.3) | 124 (48.6) | 145 (42.2) | 270 (75.6) | ||
| Hypertension | 0.86 | 0.29 | |||||
| Yes | 75 (10.7) | 47 (10.5) | 28 (10.9) | 41 (11.9) | 34 (9.5) | ||
| No | 625 (89.3) | 398 (89.4) | 227 (89.01) | 302 (88.04) | 323 (90.4) | ||
| Kidney disease | 0.71 | 0.51 | |||||
| Yes | 184 (26.3) | 119 (26.7) | 65 (25.4) | 94 (27.4) | 90 (25.2) | ||
| No | 516 (73.7) | 326 (73.2) | 190 (74.5) | 249 (72.5) | 267 (74.7) | ||
| Constipation | 0.12 | 0.11 | |||||
| Yes | 30 (4.3) | 23 (5.1) | 7 (2.7) | 21 (6.1) | 9 (2.5) | ||
| No | 670 (95.7) | 422 (94.8) | 248 (97.2) | 322 (93.8) | 348 (97.4) | ||
| History of abortion | 0.008 | 0.82 | |||||
| Yes | 102 (14.6) | 53 (11.9) | 49 (19.2) | 51 (14.8) | 51 (14.2) | ||
| No | 598 (85.4) | 392 (88.08) | 206 (80.7) | 292 (85.1) | 306 (85.7) | ||
| VDRL test | 0.81 | 0.04 | |||||
| Positive | 10 (1.4) | 6 (1.3) | 4 (1.5) | 8 (2.3) | 2 (0.5) | ||
| Negative | 690 (98.6) | 439 (98.6) | 251 (98.4) | 335 (97.6) | 355 (99.4) | ||
Clinical Factors and Associated Risk Factors for Anemia and Urinary Tract Infection Among Pregnant Women a
| Variables | OR (95% CI) | Standard Error | P-Value |
|---|---|---|---|
| Anemia | |||
| Number of received medical care (< 5 times) | 20.3 (6.7 - 61.4) | 0.28 | 0.001 |
| History of abortion | 2.6 (1.5 - 4.4) | 0.27 | 0.001 |
| UTI | |||
| History of UTI | 6.9 (2.5 - 18.8) | 0.5 | 0.001 |
Multivariable Logistic Regression for Assessing Factors Associated with Anemia, Urinary Tract Infection
5. Discussion
This study aimed to estimate the prevalence of anemia and UTI and their related factors among pregnant women in Khash city, Sistan, and Baluchestan. The prevalence of anemia among these women was high at 63.6%. A systematic review and meta-analysis estimated the prevalence of anemia in Iran at 17.9% (9). Additionally, the prevalence of anemia was reported as 44% in Golestan city (7), 17% in Bandar Abbas (10), and 14% in Tehran (11). In another study involving 2 993 pregnant Iranian women from six provinces, the overall prevalence of anemia was reported as 21.6% (12).
The primary cause of anemia in pregnant women is iron deficiency, accounting for 95% of cases, often due to insufficient iron intake and inappropriate use of iron supplements (13). This study, like others, found a significant relationship between anemia and the consumption of iron and folic acid supplements. Thus, the prevalence of anemia can be reduced through interventions such as proper nutrition education and the use of iron and folic acid supplements. Moreover, studies have shown that iron supplement use during pregnancy can increase children's birth weight (14). In this study, a higher frequency of anemia was observed in pregnant mothers who received fewer than five prenatal care visits and those who used hookah or cigarettes, highlighting the urgent need for effective public health interventions in this area. Other studies have also indicated that a lower number of prenatal visits increases the risk of anemia (15).
In studies conducted in Egypt, Pakistan, Libya, and Ethiopia, the prevalence of UTI was 32%, 23.9%, 30%, and 15.3%, respectively (3, 16-18). The combined overall prevalence of UTI among pregnant women in Khash was estimated at 49%. This higher prevalence may be attributed to the late initiation of services and the insufficient number of prenatal care visits in this city, resulting in a lack of health education for preventing infections. Furthermore, a significant portion of the women in this study lacked formal education, which correlates with a lower level of awareness about infection prevention and health-seeking behaviors, significantly impacting various aspects.
This study found a statistically significant relationship between income and UTI. Similarly, other research has indicated that low socioeconomic status is significantly associated with an increased risk of UTI (6). A study on the same topic in Pakistan by Haider et al. (19) also concluded that pregnant women with lower income levels were more prone to develop UTI than those from higher socio-economic backgrounds. Additionally, a study in Egypt by Dimetry et al. (20) concerning UTI revealed a link between low income levels and UTI. This association may stem from the relationship between socioeconomic status and factors like nutrition and body immunity, especially among pregnant women.
In this study, consistent with other research (6), the relationship between a history of urinary infections and syphilis was identified as one of the factors associated with UTIs among pregnant women. Furthermore, studies have demonstrated that sexual activity is also linked to UTI (21), as sexual intercourse can lead to bacteria being introduced into the urethra.
5.1. Conclusions
The current study reveals that the prevalence of anemia among pregnant women in Khash is significantly associated with a history of abortion, the number of prenatal care visits, the presence of thalassemia minor, the intake of iron and folic acid supplements, and smoking during pregnancy. Consequently, appropriate intervention programs, including proper nutritional education during pregnancy and the correct use of iron supplements, vitamins, and folic acid, should be organized and implemented in pregnancy or premarital clinics. Additionally, this study found that the likelihood of UTI in pregnant women was higher in the presence of risk factors such as low education level and income, a previous history of UTI, and syphilis. Therefore, pregnant women should be assessed for these relevant risk factors during their regular follow-up appointments.