1. Context
Rigidity is a prominent motor symptom in Parkinson's disease (PD), characterized by increased resistance to passive movements. When a muscle is pulled, the affected limb not only resists the motion but also remains stationary, lacking a tendency to return to its original position. This phenomenon, often referred to as plastic rigidity, can manifest in continuous (lead pipe) or intermittent (cogwheel) forms (1, 2). Parkinson's disease affects approximately 0.3% of the population in developed countries and about 1% of individuals aged 60 and older (3, 4). It typically begins after the age of 60, with the highest occurrence observed between ages 75 and 84 (5). The motor symptoms of PD usually progress slowly, at a rate of about 1.5 - 3% per year. After around six years, these symptoms tend to increase by about 22 - 40% (6).
Over the years, various objective methods and theoretical approaches have been developed to assess rigidity in Parkinson's patients. These approaches involve measuring biomechanical parameters to (MPs) gauge the degree of rigidity. The most common method in these studies is to record the resistance force during passive joint movements (2, 7, 8). This force is typically measured while applying flexion and extension motions to the target joint using a servomotor or clinician (7), (9-11), (12-14), (15-17), (18-20), (21-24) .To objectively evaluate rigidity, biomechanical parameters such as torque, angle, and electromyogram (EMG) of the relevant muscles are measured during passive joint movement (2), (8-10), (11-13), (15-17), (19-21), (22, 23), (25-27), (28-30), (31-33), (34-36), (37-39), (40-42), (7, 43, 44), (45-47), (48-51).
The Unified Parkinson Disease Rating Scale (UPDRS) is a widely used clinical assessment tool for subjectively evaluating PD symptoms. Within the UPDRS motor section (items 8 to 12), there is a rigidity score that assigns a value from 0 to 4 for the neck and each limb. A score of 0 indicates the absence of rigidity, 1 denotes slight rigidity detectable only with mirror-like or similar movements, 2 represents mild to moderate rigidity, 3 suggests marked rigidity but with an easily achievable full range of motion, and 4 indicates severe rigidity with significant difficulty in achieving a full range of motion (52).
There is some confusion regarding the recommended methods for objectively assessing rigidity in Parkinson's patients.
2. Objectives
The purpose of this scoping review is to identify, classify, and compare biomechanical objective outcome measures (BOM) proposed between 1980 and the end of 2023.
3. Evidence Acquisition
3.1. Protocol and Registration
A scoping review was conducted on studies evaluating rigidity, following the methodological recommendations of the Joanna Briggs Institute for systematic scoping reviews (53). The questions addressed in this paper were:
1. What biomechanical parameters have been proposed for assessing rigidity in PD between 1980 and 2023?
2. What are the electrophysiological, kinematic, and kinetic parameters used in the assessment of rigidity, and how are they categorized?
3. Which clinical scales or assessments are commonly associated with the biomechanical parameters for rigidity evaluation?
Studies were included in the review if they met the following criteria:
1. Research focusing on the assessment of rigidity in individuals diagnosed with PD.
2. Participants diagnosed with PD of any gender and age.
3. Randomized clinical trials, randomized controlled trials, clinical trials, case reports, or cohort studies.
4. Complete text presented in the English language.
The exclusion criteria consisted of the following:
1. Studies lacking completeness or results presentation.
2. Studies that included non-human subjects.
3. Studies focusing on the medical and neurological aspects of rigidity.
4. Studies discussing rigidity assessment in individuals with PD but deviating from the primary focus.
The search methodology followed the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines (54). This included various scientific databases such as Google Scholar, PubMed, Science Direct, Springer, and Civilica. Specific keywords and queries ("Parkinson AND Rigidity" AND "Parkinson disease OR Rigidity") were used across paper titles, abstracts, and keywords, spanning from 1980 to the end of 2023. Notably, Science Direct was searched from 1995 to 2023 due to time limitations.
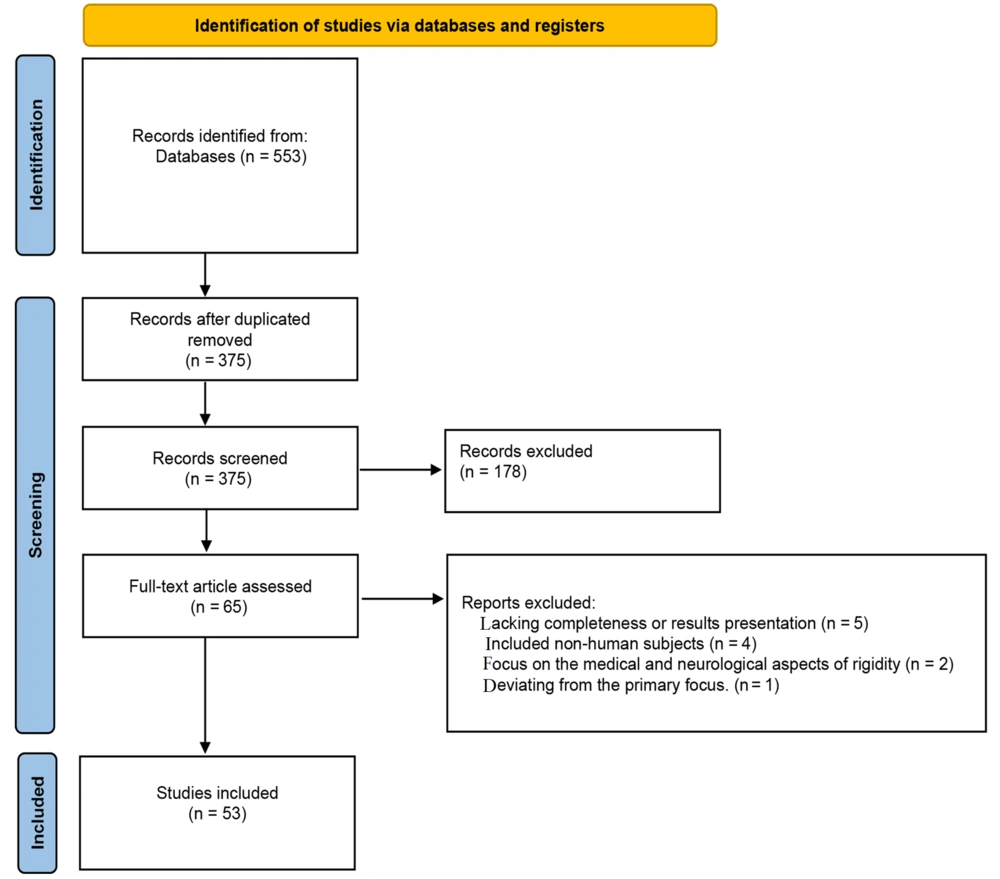
After removing duplicate articles, we identified 553 unique articles based on their titles and abstracts. We then excluded articles that were not published in English or lacked full-text availability, resulting in 375 articles for further assessment. These articles were further filtered to exclude studies not related to biomechanics or those not involving human subjects, leaving us with 65 relevant articles.
A thorough review of these 65 articles by authors S. Asghari and M. R. Azghani resulted in the exclusion of 12 papers based on the established criteria, leaving 53 studies for classification (Figure 1).
Our primary objective was to compile suggested rigidity assessment measures (Biomechanical Objective Measures-BOMs), categorized into three types: Electrophysiological (MP1), kinematic (MP2), and kinetic (MP3) parameters. These measures were evaluated against the UPDRS rigidity score as the primary benchmark.
The selection and analysis of the 53 studies were carried out in two phases by the authors (S. Asghari and M. R. Azghani). In cases where consensus could not be reached, a third reviewer (F. Rahimi) provided the final decision. In the initial phase, these studies were analyzed for demographic information, including author (year), subject demographics, medication status, joint condition, and clinical tests (Table 1). In the second phase, the studies were categorized based on laboratory conditions, data type (velocity, amplitude), device type, joint, and muscle (Table 2). Finally, the extraction and evaluation of BOMs, experimental conditions, and clinical scales used in these studies were conducted by the first and second authors to derive overall results (Table 3).
| Author and Reference (y) | Subject | Medication Statue | Joint Statue | |||
|---|---|---|---|---|---|---|
| Control | Parkinson | On | Off | Active | Passive | |
| Marusiak et al. (2010) (38) | 10 | 8 | * | - | - | * |
| Xia et al. (2006) (22) | 7 | 12 | * | * | - | * |
| Endo et al. (2009) (47) | 24 | 27 | - | * | - | * |
| Xia et al. (2009) (17) | - | 12 | * | * | - | * |
| Mak et al. (2007) (29) | G1:6; G2:15 | G1:6; G2:15 | * | * | - | * |
| Teravainen et al. (1989) (16) | 12 | 29 | - | - | - | * |
| Levin et al. (2009) (9) | 8 | 15 | * | - | * | - |
| Little et al. (2012) (32) | - | 12 | * | - | * | * |
| Hong et al. (2007) (49) | 22 | 12 | * | - | - | * |
| Xia et al. (2011) (23) | - | 17 | * | * | - | * |
| Robichaud et al. (2004) (41) | 12 | 12 | * | * | * | * |
| Fung et al. (2000) (30) | 10 | 20 | * | * | * | * |
| Xia and Rymer (2004) (2) | - | 6 | * | * | - | * |
| Shapiro et al. (2007) (50) | 10 | 10 | * | * | * | * |
| Park et al. (2011) (7) | 45 | 12 | * | - | - | * |
| Caligiuri (1994) (20) | 25 | 29 | * | - | * | * |
| Kwon et al. (2015) (44) | 18 | 19 | - | - | - | * |
| Prochazka et al. (1997) (21) | - | 14 | * | - | - | * |
| Sepehri et al. (2007) (19) | 11 | 41 | * | * | - | * |
| Powell et al. (2011) (45) | - | 8 | * | * | - | * |
| Powell et al. (2012) (15) | 8 | 12 | * | * | * | * |
| Endo et al. (2012) (55) | - | 5 | * | * | * | * |
| Oladi et al. (2017) (56) | 4 | 2 | - | - | - | * |
| Powell et al. (2017) (8) | - | 12 | * | * | - | * |
| Bergui et al. (1992) (25) | 14 | - | - | * | * | |
| Mera and Cody. (1993) (28) | 19 | 20 | * | * | - | * |
| Watt et al. (1986) (57) | - | 11 | * | - | - | * |
| Webster. (1959) (36) | 1 | 1 | * | - | - | * |
| Patrick et al. (2001) (10) | - | 4 | - | * | - | * |
| Mera and Cody. (1992) (34) | 10 | 9 | * | * | * | * |
| Marusiak et al. (2012) (35) | - | 10 | * | * | - | * |
| Zetterberg et al. (2014) (13) | 14 | 25 | - | * | * | * |
| Cano de la cureda et al. (2010) (46) | - | 36 | * | - | - | * |
| Van Emmerik et al. (1999) (43) | 11 | 27 | - | * | * | - |
| Cantello et al. (1995) (27) | 8 | 8 | * | - | - | * |
| Bartolic et al. (2005) (40) | - | 8 | * | * | * | - |
| Lee et al. (2002) (12) | 12 | 16 | - | * | - | * |
| Kwon et al. (2014) (58) | - | 8 | - | * | * | - |
| Duval et al. (2002) (39) | - | 30 | * | - | - | * |
| Tabbel et al. (2008) (11) | - | 52 | * | - | - | * |
| Ratsep and Asser (2019) (2017) (37) | 15 | 15 | * | * | - | * |
| Perera et al. (2019) (42) | 16 | 8 | * | - | - | * |
| Xia et al. (2016) (59) | 14 | 14 | * | * | - | * |
| Costa et al.(2015) (60) | - | 6 | - | * | * | - |
| Zito et al. (2018) (61) | 12 | 4 | - | - | - | * |
| Nuyens et al. (2000) (31) | 10 | 10 | - | * | - | * |
| Relja et al. (1996) (62) | 103 | 24 | * | - | - | * |
| Anastasopoulos et al. (2009) (63) | 23 | 14 | * | - | - | * |
| Solopova et al. (2014) (64) | 22 | 25 | * | * | - | * |
| Marusiak et al. (2018) (65) | 10 | 8 | * | - | * | * |
| Kirollos et al. (1996) (14) | 2 YHC; 2 EHC; 2 EAP | 2 | * | * | * | - |
| Endo et al. (2015) (18) | - | - | * | - | - | * |
| Asci et al. (2023) (24) | 25 | 20 | * | - | * | - |
Demographic Information of the Reviewed Papers
| Author and Reference (y) | Device | Joint and Muscle | Data Type | Data Distribution | Velocity, ᵒ/s | Amplitude, ᵒ |
|---|---|---|---|---|---|---|
| Marusiak et al. (2010) (38) | Myoton-3, electromyogram (EMG), mechanomyogram | Biceps brachii (BB) muscle | CAT_3 | Normal | - | - |
| Xia et al. (2006) (22) | Emulated encoder, strain gauge torque transducer, servomotor | Wrist | CAT_2 | Normal | 50 | ± 30 |
| Endo et al. (2009) (47) | Three-axis force sensors, gyroscope, EMG | Elbow; BB; triceps brachii (TB) | CAT_3 | Normal | - | 10 - 110 |
| Xia et al. (2009) (17) | Emulated encoder, strain gauge torque, transducer, servomotor, EMG | wrist flexure and extensor wrist muscle | CAT_3 | Normal | 50, 280 | ± 30 |
| Mak et al. (2007) (29) | Cybex @ norm isokinetic dynamometer | Trunk | CAT_2 | Normal | 60 | 60, 75, 90, and 105 |
| Teravainen et al. (1989) (16) | Torque motor with position feedback | Wrist | CAT_2 | Normal | 12 - 240 | ± 15 and ± 30 |
| Levin et al. (2009) (9) | Goniometer, EMG | Elbow, BB, and TB | CAT_3 | Normal | - | 90 |
| Little et al. (2012) (32) | Goniometer strain gauge | Wrist | CAT_2 | Normal | - | - |
| Hong et al. (2007) (49) | Rigidity analyzer | Elbow | CAT_2 | Normal | - | Full flexion extension |
| Xia et al. (2011) (23) | Emulated encoder, strain gauge torque transducer, servomotor, EMG | Wrist flexure and extensor muscle of finger | CAT_3 | Normal | 50 | 60 |
| Robichaud et al. (2004) (41) | Strain gauge torque, transducer, EMG, capacitive transducer | Elbow, BB, and TB | CAT_3 | Normal | - | 90 |
| Fung et al. (2000) (30) | Potentiometer, torque motor, EMG | Wrist | CAT_3 | Not normal | - | ± 30 |
| Xia and Rymer (2004) (2) | Emulated encoder, strain gauge torque, transducer, servomotor, EMG | Wrist | CAT_2 | Normal | 50 | ± 30 |
| Shapiro et al. (2007) (50) | Capacitive transducer, servo control, EMG | Elbow, TB, BB | CAT_3 | Normal | - | ± 30 |
| Park et al. (2011) (7) | Potentiometer, bidirectional load cell, accelerometer | Wrist | CAT_2 | Normal | - | -35 + 55 |
| Michael P. Caligiuri (1994) (20) | Transducer, strain gauges | Wrist | CAT_2 | Not normal | - | ± 45 |
| Kwon et al. (2015) (44) | EMG, encoder, servomotor | Wrist flexor and extensor wrist muscle | CAT_1 | Not normal | 30, 50 | 60 |
| Prochazka et al. (1997) (21) | Force-gauge, length-gauge | Elbow | CAT_2 | Normal | - | - |
| Sepehri et al. (2007) (19) | Balanced strain gage force transducer, potentiometer, EMG | Elbow, BB, TB | CAT_3 | Normal | - | - |
| Powell et al. (2011) (45) | Emulated encoder, strain gauge torque, transducer, servomotor, EMG | Wrist, FCR, FCU, FDS, ECR, ECU, EDC | CAT_3 | Normal | 50, 280 | ± 30 and ± 60 |
| Powell et al. (2012) (15) | Emulated encoder, strain gauge torque, transducer, servomotor, electromyogram (EMG) | Wrist, FCR, FCU, FDS, ECR, ECU, EDC | CAT_3 | Normal | 50 | ± 30 |
| Endo et al. (2012) (55) | Force sensor, gyroscope, EMG, electromagnetic sensor | Wrist BB, TB | CAT_3 | Normal | - | 30, 60, 90 |
| Oladi et al. (2017) (56) | L6D load cell, strain gauge, Potentiometer | Elbow | CAT_2 | Normal | - | 120 |
| Powell et al. (2017) (8) | Emulated encoder, strain gauge torque, transducer, servomotorelectromyogram | Wrist, FCR, FCU, FDS, ECR, ECU, EDC | CAT_3 | Normal | 50 | 60 |
| Bergui et al. (1992) (25) | Potentiometer, EMG, oscilloscope | Knee quadriceps femoris | CAT_1 | Normal | 100, 200 | 10 |
| Mera and Cody. (1993) (28) | Electromagnetic vibrator, Servo control, EMG | Wrist flexor carpi radialis (FCR) | CAT_1 | Normal | - | - |
| Watt et al. (1986) (57) | Torque motor, EMG, potentiometer | Elbow, TB | CAT_3 | Normal | - | - |
| Webster.(1959) (36) | Turntable and torque detecting system | Wrist | CAT_2 | Normal | 1.25 | 100 |
| Patrick et al. (2001) (10) | Force transducer, piezoelectric gyroscope | Elbow | CAT_2 | Non-normal | - | - |
| Mera and Cody. (1992) (28) | Accelerometer, amplifier, transducer, camera, EMG | Wrist, FCR | CAT_2 | Normal | - | 90 |
| Marusiak et al. (2012) (35) | Myoton-3, EMG | TB, BB, BR | CAT_3 | Normal | - | - |
| Zetterberg et al. (2014) (13) | Neuro flexure | Wrist | CAT_2 | Non-normal | 5, 236 | 50 |
| Cano de la Cureda et al. (2010) (46) | Isokinetic dynamometer | Trunk | CAT_2 | Normal | 30, 45, 60 | 50 |
| Emmerik et al. (1999) (43) | Optoelectronic tracking device, treadmill, sell spot camera | Pelvic and thoracic | CAT_2 | Normal | 0.2 - 1.4, 1.4 - 0.2 | - |
| Cantello et al. (1995) (27) | EMG, accelerometer, oscilloscope | FDI muscle | CAT_1 | Normal | - | - |
| Bartolic et al. (2005) (40) | Force plate | Forward-backward and side-to-side body oscillations | CAT_2 | Normal | - | - |
| Lee et al. (2002) (12) | Encoder, torque sensor, EMG | Elbow, BB, TB | CAT_3 | Normal | 40, 80, 120, 160 | 75 |
| Kwon et al. (2014) (58) | Bi-directional load cell, potentiometer, accelerometer | Wrist | CAT_2 | Normal | - | - |
| Duval et al. (2002) (39) | Goniometer, EMG | Extensor digitorum communis, flexor carpi radialis | CAT_1 | Normal | - | 60 |
| Tabbel et al. (2008) (11) | Rigidity analyzer | Elbow | CAT_2 | Normal | - | - |
| Ratsep and Asser (2019) (37) | Myoton-3 | Elbow musculus extensor digitorum | CAT_3 | Normal | - | - |
| Perera et al. (2019) (42) | BiRD | Metacarpophalangeal joint | CAT_2 | Normal | - | 45 |
| Xia et al. (2016) (59) | EMG, parallel-cascade system, strain gauge torque, transducer | Wrist FCR, FCU, FDS, ECR, ECU, EDC, bellis of wrist | CAT_3 | Normal | - | ± 2 |
| Costa et al.(2015) (60) | Microcontroller, gyroscope, kino accelerometer, magnetometer | wrist | CAT_2 | Normal | - | - |
| Zito et al. (2018) (61) | Torque sensor, optical encoder, gearbox, servo motor, potentiometer, I/O card (wrist resistance robot) | wrist | CAT_2 | Non-normal | 10, 50, 100 | -60 to +60 |
| Nuyens et al. (2000) (31) | Electric servomotor, strain gauge bridge torque meter | knee | CAT_2 | Normal | 60,180,300 | - |
| Relja et al. (1996) (62) | Tonometer (torque and angle transducer) | Elbow | CAT_2 | Non-normal | - | 0 to 53 |
| Anastasopoulos et al. (2009) (63) | Bárány chair, a device for measuring horizontal head torque | Neck | CAT_2 | Normal | 2.3,4.6,18.4 | |
| Solopova et al. (2014) (64) | Potentiometer, strain gauge, EMG | Knee, hip, ankle | CAT_3 | Normal | 7 | 10 for dorsiflexion, other remains 20 |
| Marusiak et al. (2018) (65) | Myoton-3 device | Elbow (BB, TB) | CAT_3 | Normal | - | - |
| Kirollos et al. (1996) (14) | Servomotor, semiconductor strain gauge, potentiometer | Elbow | CAT_2 | Normal | - | - |
| Endo et al (2015) (18) | 3-axis force sensors, gyro sensor | Elbow | CAT_2 | Normal | 60,120 | 10-110 |
| Asci et al. (2023) (24) | Robot-assisted wrist extensions | wrist | CAT_1 | Normal | 7 different angular velocities | - |
Summary Method and Experimental Condition in the Reviewed Papers
| Author and Reference (y) | Experimental Condition | Outcome Measures (BOM) | Clinical Scale | Result |
|---|---|---|---|---|
| Marusiak et al. (2010) (38) | Rigidity Unified Parkinson Disease Rating Scale (UPDRS) | Electromyography (EMG) and mechanomyography signals, amplitude, myotonometry Signals | - | The myotonometer, a remarkably sensitive tool, detects heightened muscle passive stiffness in Parkinson's patients compared to those without the condition. |
| Xia et al. (2006) (22) | Medication state (on/off), movement direction (flexion, extension) | Temporal score (Nm.s), work score (Nm.deg), torque-angle slope (mNm/°) | H&Y, rigidity UPDRS | During the 'off' state, extension resistance was notably greater than flexion. Dopaminergic treatment reduced the time needed for extension and the effort required for both movements. Patients in the 'on' state scored higher than the control group. |
| Endo et al. (2009) (47) | Rigidity UPDRS | Elastic coefficients (in extension and flexion), the sum of the difference of Bias, and EMG Index (for BB and TB) | Rigidity UPDRS | The combined elastic coefficients, represented by the 'Bias Difference' and 'EMG Index' for the biceps brachii, showed strong correlations with the UPDRS score. |
| Xia et al. (2009) (17) | Medication statue (on/off), velocity, stretch reflex, and shortening reaction | Objective rigidity (OR) scores, normalized EMG, normalized EMG activations in the stretched muscles | Rigidity UPDRS | Objective rigidity scores showed a stronger correlation with EMG ratios than isolated EMG measurements of stretched muscles. A significant correlation was observed between the OR score and EMG ratio during rapid extension without medication. |
| Mak et al. (2007) (29) | Velocity, Parkinsonian/control, muscle tone | TourqePF, tourqepe, work was done, functional reach distance | Rigidity UPDRS | - Work and peak resistance torque for passive trunk flexion, and extension were consistently reliable. - Parkinson's disease (PD) patients showed increased muscle tone, with higher work and resistance torques at faster movement speeds. - The rise in trunk muscle tone with increased movement speed was more pronounced in PD patients. |
| Teravainen et al. (1989) (16) | Velocity, amplitude, frequency, clinical rigidity score | Objective rigidity score (ORS) | H&Y | Angular velocities of 140 - 190 degrees/second and ± 25 ± 30 degrees displacements are highly effective in detecting Parkinsonian rigidity, correlating well with the CRS. |
| Levin et al. (2009) (9) | Rigidity UPDRS, DBS_STN off/on | Average EMG activity (biceps, triceps), angular velocity (extension-flexion) | Rigidity UPDRS | The EMG patterns in elbow joint extension and flexion movements show a significant correlation with clinical assessments. |
| Little et al. (2012) (32) | Medication statue (on/off), frequency | Elastic coefficient | Rigidity UPDRS | Low-frequency 20 Hz stimulation increased rigidity by 24%, while high-frequency 130 Hz stimulation reduced it by 18%. Low-frequency stimulation (5, 10, 20 Hz) had consistent effects in both flexion and extension. |
| Hong et al. (2007) (49) | Activation condition (BASELINE, IPSI, CONTRA, BILAT 2Hz, BILAT 1 Hz) | Displacement, elastic and viscous stiffness, impedance | Rigidity UPDRS | Movement of either the opposite or same-side lower limb can heighten arm rigidity in individuals with PD, but the effects of left and right movements do not combine. |
| Xia et al. (2011) (23) | Medication state (off vs. on), the direction of movement (flexion vs. extension), muscle group (shortening muscles vs. lengthening muscles) | The slope of torque-angle plots, EMG recordings during passive Flexion, Extension | H&Y, rigidity UPDRS, III UPDRS | In flexion, the slopes for shortening reaction (SR) were notably smaller compared to SII, while in extension, the slopes for SII were significantly reduced. |
| Robichaud et al. (2004) (41) | Disease (healthy, Parkinson), medication status (on, off), muscle group (flexure, extensor), target distance (36ᵒ,54ᵒ,72ᵒ) | Peak velocity, duration of the first agonist burst, Qag1/T, the magnitude of the first agonist burst, number of agonist bursts before peak velocity, frequency of agonist bursting, cant, the latency of the main antagonist burst, Co-Contraction Index | Rigidity UPDRS | Extension movements are notably more impaired than flexion movements in PD. |
| Fung et al. (2000) (30) | Clinical rigidity scores, joint condition (rest, active), frequency | Angular impulse, work scores | H&Y, III UPDRS | Angular impulse serves as a reliable, objective indicator of Parkinsonian rigidity. Activation augments rigidity, with varying effects among patients. |
| Xia et al. (2004) (2) | Medication state (off vs. on), the direction of movement (flexion vs. extension) | The slope of torque-angle plots, normalized EMG | H&Y, rigidity UPDRS | There was a robust correlation between SR and the torque-angle slope in the absence of medication. |
| Shapiro et al. (2007) (50) | Medication state (off vs. on), DBS_STN off/on, joint condition (passive, active) | Work scores, EMG, rigidity UPDRS | H&Y, rigidity UPDRS | Deep brain stimulation (DBS) of the subthalamic nucleus (STN) may provide superior relief from upper limb rigidity in PD patients compared to pre-surgery medication doses. |
| Park et al. (2011) (7) | Rigidity UPDRS | Damping constant, elastic constant, Offset torque, normalized work, impulse per cycle | Rigidity UPDRS | Mean viscosity is a better representation of clinical rigidity scores in both flexion and extension. Work and impulse are linked to clinical rigidity scores. Model 1's viscosity is suitable for clinical rigidity quantification, while Model 2's viscosity aids in distinguishing PD and studying phase-related characteristics of rigidity. |
| Michael P. Caligiuri (1994) (20) | Joint condition (passive, active) | Stiffness | H&Y | The OR score, reflecting how motor activity impacts wrist muscle stiffness, closely correlates with clinical Parkinsonian rigidity ratings. |
| Kwon et al. (2015) (44) | Clinical rigidity score, patient group (PD, control), muscle group (FCR, FCU, ECR, ECU), different phase (stretching phase and shortening phase) | RMS EMG, MS EMG | Rigidity UPDRS | MSSS EMG ratios were consistently below one in all muscle groups. Wrist muscles exhibit higher average EMG activity during shortening compared to stretching. Parkinsonian rigidity torque resistance is not merely the sum of independent, opposing torque pairs. |
| Prochazka et al. (1997) (21) | Rigidity UPDRS | Mechanical impedance | H&Y, rigidity UPDRS | A newly available device for quantifying clinical rigidity assessments promises to usher in standardized rating protocols for rigidity in the near future. |
| Sepehri et al. (2007) (19) | Patient group (PD, control), gender (male, female), body side (right or left), UPDRS scales with reinforcement/without reinforcement | Total slope (TS), total hysteresis (TH), range of motion of the joint (ROM), normalized total Hysteresis (NTH) | Rigidity UPDRS | Correlation between the viscous component of stiffness and UPDRS scores compared to the elastic component. |
| Powell et al. (2011) (45) | Velocity, amplitude, medication state (Off vs. On), UPDRS rigidity, Wrist condition (rest, active) | Work, angular impulse, torque-angle slope, EMG variable | Rigidity UPDRS | Higher displacement amplitude and velocity were linked to significantly increased rigidity, elevated EMG ratio, and mean EMG in stretched muscles, as indicated by work scores and angular impulses. The use of dopaminergic medication did not lead to a reduction in rigidity. |
| Powell et al. (2012) (15) | Medication state (off vs. on), joint condition of the contralateral joint (passive, active), the direction of movement (flexion, extension) | Work, mean amplitude of EMG | Rigidity UPDRS | There was a notable increase in torque resistance in the off-med state compared to healthy individuals and the on-med state. In the active condition, the differences in torque resistance became more pronounced. Medication significantly reduced the gap in torque resistance between PD patients and healthy controls in both passive and active conditions. |
| Endo et al. (2012) (55) | The direction of movement (flexion, extension), DBS-on, off | Elastic coefficient (flexion, extension), sum of the differences in averaged torque values, finger-tapping interval (FTI), maximum opening velocity (MoV), maximum closing velocity (McV), Maximum amplitude (MA), Standard deviation (SD) FTI, the frequency was the number of fingers taps in 15 s (NFT). | Rigidity UPDRS | Among the three parameters, the discrepancy in average torque values proves to be the most responsive. Following deep brain stimulation (DBS), there was a significant enhancement in three parameters: the mean of MOV, the mean of McV, and the mean of MA. |
| Oladi et al. (2017) (56) | Rigidity UPDRS | Normalized flexion Hysteresis (NFH), normalized extension Hysteresis (NEH), normalized dead-zone Hysteresis (NDZH), normalized total Hysteresis (NTH), range of motion (ROM) | Rigidity UPDRS, UPDRS III | The proximity of quantitative and qualitative results and validation of the device |
| Powell et al. (2017) (8) | Three normalization techniques (RAW, DIV, MINUS), The direction of movement (flexion, extension), medication state (off vs. on) | Normalized mean EMG of extensors during the flexion movement, The average of normalized mean EMG of flexors during the extension movement, work, torque–angle slope | Rigidity UPDRS | Using EMG normalization techniques can complicate the analysis of changed reflex responses in PD patients after taking dopaminergic medication. |
| Bergui et al. (1992) (25) | Rigidity UPDRS, reflex size (normal, increased), test condition (rest, background activity) | EMG value | Rigidity UPDRS, UPDRS III | The link between reflex size and rigidity was examined. Parkinson's disease patients exhibited a reduced threshold for the reflex during resting trials, and long-latency reflexes displayed increased size and duration in trials with background activity. |
| Mera and Cody. (1993) (28) | Medication state (off vs. on), M1 (20 – 40 ms), M2 (50 – 90 ms) Phases | EMG value of FCR muscle | Rigidity UPDRS | The M2 stretch reflexes in the flexor carpi radialis (FCR) are amplified in PD. The heightened M2 reflex activity, as assessed by reflex function tests, is not the sole or direct cause of wrist rigidity in PD. |
| Watt et al. (1986) (57) | Clinical tone at the time of testing | Compliance (°/Nm), stiffness (Nm/radian), muscle volume | Webster disability grading | Alterations in the passive mechanical characteristics of the upper limb in Parkinsonian rigidity may explain the increased flexion in the neutral elbow angle and heightened passive stiffness. |
| Webster.(1959) (36) | Velocity, Amplitude | Work, displacement force | H&Y | High sensitivity to the Work in compression, the other parameter |
| Patrick et al. (2001) (10) | Velocity, frequency, amplitude, clinical rigidity score, medication status (on, off), the direction of movement (flexion, extension) | Mechanical impedance | - | Mechanical impedance was nonlinearly related to UPDRS ratings of rigidity at the elbow and wrist. |
| Mera and Cody. (1992) (34) | Stretch reflex, the direction of movement (flexion, extension), medication statute (on, off), joint condition (rest, activation) | Displacement, normalized EMG (flexion, extension) | Rigidity UPDRS | Enhancement of stretch reflex activity has a significant role in the genesis of Parkinsonian rigidity |
| Marusiak et al. (2012) (35) | Medication status (on, off), UPDRS | RMS_EMG, the amplitude of EMG (BB, TB, BR), S-MYO (BB, TB, BR) | Webster clinical scale | Reduced myometric stiffness and EMG amplitude in all tested muscles, along with lower clinical rigidity scores, were observed during the medication on-phase in comparison to the off-phase. |
| Zetterberg et al. (2014) (13) | Velocity (fast, Slow), group of subject (PD, healthy), UPDRS, joint condition (rest, active) | Inertia component (IC), viscous component (VC), neural component (NC), elastic component (EC) | H&Y, rigidity UPDRS | Reflex activity from stretching, not nonneural resistance, is the primary factor in wrist muscle rigidity in PD. The Neuroflexor device has the potential to be a valuable tool for rigidity quantification in clinical and research settings. |
| Cano de la cureda et al. (2010) (46) | The severity of disease, disease duration, functional status scales, velocity (30,45,60), movement direction (flexion, extension), HRQoL | Work was done | UPDRS, H&Y | Functional status was linked to trunk extensor rigidity across all angular velocities. Axial motor impairments affect the quality of life (QoL) and functional status in PD patients. Trunk rigidity correlated with HRQoL. |
| Emmerik et al. (1999) (43) | The direction of increasing and decreasing velocity, effect of the seventh stage velocity, healthy and PD | Stride duration, angular rotations of pelvic and thorax, relative phase analysis | H&Y | The PD group exhibited significantly reduced alterations in the mean relative phase and less variability in the relative phase in terms of coordination between transversal pelvic and thoracic rotations. |
| Cantello et al. (1995) (27) | Clinical University Colombia Scales (CURS) | Average value of EMG at rest FDI | H&Y, UPDRS III | At "rest," EMG activity primarily consists of low-threshold motor unit discharges, following a recruitment pattern akin to that induced by descending corticospinal signals. |
| Bartoli et al.(2005) (40) | Frequency (0.2 - 10), medication status (on, off), UPDRS | Amplitude | Columbia University Rating Scales | Improvement in Postural stability in PD with descries in rigidity |
| Lee et al. (2002) (12) | Velocity position | Averaged speed-dependent reflex torque (ASRT), Velocity sensitivity of ASRT (VASRT), segmented ASRT (SASRT) | H&Y, UPDRS III | Velocity dependence analysis indicates that rigidity and spasticity have approximately equal velocity-dependent properties. |
| Kwon et al. (2014) (58) | Clinical scales, DBS (on, off) | Elastic coefficient, work, impulse, mechanical impedance | Rigidity UPDRS | Correlation coefficients between mechanical measures and clinical scores for multiple settings (averaged for 14 limbs) were, and the best correlation is shown for viscosity. |
| Duval et al. (2002) (39) | Side (ips, contra), position (sitting, supine), test (pretest, posttest) | Index subtracted from EMG | Rigidity UPDRS | It is possible to modify the level of evoked stretch responses by using Trager therapy. |
| Tabbel et al. (2008) (11) | DBS condition (BOTH on, BOTH off, left on, right on) (IPSI on, contra on) | Force displacement, arm length, Impedance | H&Y, rigidity UPDRS | Bilateral STN DBS significantly improved rigidity and bradykinesia with contralateral, ipsilateral, and bilateral stimulation. Bilateral stimulation was more effective at reducing rigidity than unilateral stimulation, with no significant difference between ipsilateral and contralateral stimulation. Contralateral stimulation significantly improved hand rotation speed over ipsilateral stimulation. All stimulation conditions improved walking time, with bilateral stimulation providing the most significant improvement. |
| Ratsep et al. (2017) (37) | Before, after PWM, DBS on, off, High, Low Rigidity | Viscoelastic stiffness (N/m) | UPDRS III | Wrist rigidity clinical scores improved from 3.0 (on a scale of 1 - 4) to 0.93 (on a scale of 0 - 2). Stimulation-on conditions were only significant when measurements were taken after passive wrist movements. |
| Perera et al. (2019) (42) | Movement direction (flexion, extension), DBS (on, off), activation maneuver, MDS-UPDRS (PD, healthy) | Force rate, peak force, work estimate, charge | H&Y, UPDRS | Our instrument's rigidity measurements exhibited moderate agreement with the MDS-UPDRS and displayed variations among therapeutic states, activation conditions, and patient/healthy groups. |
| Xia et al. (2016) (59) | Between subject fixed effect (neural, nonneural), medication state (on, off), disease state (healthy, Parkinson) | Torque, EMG amplitude, joint position | MDS-UPDRS | Parkinson's disease patients exhibit greater neural reflexes and intrinsic mechanical factors contributing to rigidity compared to healthy controls. Dopaminergic medication reduces the influence of the neural reflex component on rigidity while leaving the inherent muscle mechanical component unaffected. |
| Costa et al. (2015) (60) | DBS state (on, off) | average angular speed, average peak value, signal descriptor ϕ | H&Y, rigidity UPDRS | The descriptor distinguished between non-rigid and rigid states, correctly identifying 83.9% of signals compared to the agreement of two specialists. A sensitive methodology for cogwheel rigidity detection from angular speed signals was developed with a sensitivity of 0.93. |
| Zito et al. (2018) (61) | Disease state (healthy, Parkinson), velocity (10, 50, 100) | Torque, velocity, position | - | Whole-body rigidity ratio (WRR) effectively distinguishes PD patients' rigidity from the normal muscle tone in healthy controls (HC) across various velocities. The primary factor contributing to rigidity in PD is an elevation in passive movement resistance caused by stretch reflex activity. |
| Nuyens et al. (2000) (31) | Velocity (60, 180, 300), movement direction (flexion, extension) | Torque, EMG activity | H&Y, UPDRS, Schwab & England Barthel Index | The velocity and course of motion exerted a more pronounced influence on stiffness during the extension stage as opposed to flexion. |
| Relja et al. (1996) (62) | Movement direction (flexion, extension), medication statue, basal and activated statue | Work | UPDRS, H&Y | Measured rigidity is significantly greater in PD compared to the control group. Rigidity inactivation is notably enhanced exclusively in PD patients. Activated rigidity is lower in the control group compared to PD. Basal and activated rigidity decrease in the on state compared to the off state. |
| Anastasopoulos et al. (2009) (63) | Rotation phase (head-only, trunk-only, head trunk rotation), angular velocity (2.3,4.6,18.4) | Peak torque | UPDRS, rigidity UPDRS, H&Y | Greater peak torque for PD vs. control |
| Solopova et al. (2014) (64) | Medication state (on, off), movement direction | The resistance force, shortening reaction (mean value of EMG values eliminating the basal activity in each test), the latency period of the test | H&Y | Hip muscle stiffness is significantly higher in PD patients (1.5 - 1.7 times for flexors and extensors) than in controls. - Flexors exhibit greater rigidity than extensors at the hip and knee joints (1.5 - 1.6 times). - People with PD have 1.3 times more rigidity in ankle dorsiflexion compared to controls. Plantar flexors are more rigid than dorsal flexors in PD patients. - Medication improves hip and knee stiffness but doesn't affect ankle stiffness. |
| Marusiak et al. (2018) (65) | Rest and 10% of the subject’s maximal voluntary contraction (MVC), myotonometry record times (3, 5, 10, 15, 20) | Muscle stiffness [S-MYO (N/m)] | - | High reliability in myotonometry record in rest and 10% MVC |
| Kirollos et al. (1996) (14) | Test condition (baseline, activation, or recovery), medication state | Mean work | - | Higher work values by a unit of displacement for PD patients |
| Endo et al. (2015) (18) | Movement direction (flexion, extension), velocity | The sum of the differences in bias, Elastic coefficient during flexion, and extension | UPDRS III | Velocity dependence was not observed for the elastic coefficient. Velocity-dependent behavior was evident in the bias difference. |
| Asci et al. (2023) (24) | Wrist extensions at different angular velocities | Biomechanical measures (elastic, viscous, and neural components), neurophysiological measures (short- and long-latency reflex and shortening reaction) | UPDRS III | In PD, the meas urable stiffness, the velocity-dependent characteristic of stiffness, heightened long-delay reflexes, and the association between biomechanical and neurophysiological irregularities with the clinical assessment of stiffness. |
Classification of Experimental Conditions and Outcome Measures (BOMs) of the Reviewed Papers
This structured approach allowed us to categorize the literature on biomechanical objective BOM for rigidity assessment in PD, providing valuable insights into the current state of research in this area.
4. Results
All 53 examined studies are presented in Table 1. These studies assessed either both Parkinson’s patients and HC (35 studies) (7, 9, 13, 14), (19, 20, 22, 24) , (27-29), (30, 31, 33), (34, 36, 37), (38, 41-43), (45, 47, 49), (12, 50, 56), (59, 61, 62), (63-65). or only PD patients (17 studies) (2, 8, 10), (11, 15, 17, 18), (21, 23, 32), (35, 39, 40), (46, 51, 55), (58, 60). The medication state during rigidity assessment (on/off) is shown in the table, although it was not reported in 5 studies (16, 25, 44), (56, 61).
For rigidity assessment, 35 studies used the passive movement of the joint of interest (2, 7, 8), (10, 11, 16), (17-19), (21, 22, 27), (28, 29, 31), (35-37), (38, 39, 42), (44-46), (47, 49, 51), (12, 56, 59), (61-64, 66); 7 studies asked participants to move their joint actively (9, 14, 33), (40, 43, 60); and 11 studies examined participants under both types of movement (active and passive) (13, 20, 25), (30, 32, 34), (41, 45, 50), (55, 65).
The studies were categorized based on their mechanical parameters (MP1-MP3). To differentiate the approaches for objective assessment of rigidity, we identified three distinct categories:
- CAT-1 studies use only MP1 parameters (25, 27, 28), (34, 40, 58).
- CAT-2 studies use both MP2 and MP3 parameters (2, 7, 10), (11, 13, 14), (16, 18, 20),(21, 22, 29), (32, 36, 40), (42, 43, 46), (49, 56, 61), (58, 60, 62, 63).
- CAT-3 studies use all MPs for rigidity evaluation (Table 2) (8,15,17,19,30,31,35,37,38,41,46,48,51,52,57,58,62–64,67).
Table 2 also indicates the joint for which rigidity was examined. Twenty-three studies assessed rigidity at the wrist joint (2, 7, 8), (13, 15, 16), (17, 20, 22), (23, 24, 28), (30, 32, 34), (36, 44, 45), (55, 59, 61), (58, 60), 17 at the elbow joint (9, 11, 14), (18, 19, 21), (36, 37, 41), (12, 47, 49-51, 56), (62, 65), 3 in the trunk (29, 40, 46), 3 in the lower limbs (knee, hip, and ankle) (25, 31, 64), and one in the neck (63). The remaining 7 studies examined muscle activities in addition to the related joint movements (27, 35, 38), (39, 42, 43).
The muscles for which EMG recordings were performed included the biceps brachii (BB) and triceps brachii (TB) muscles (9, 19, 35), (35, 38, 41), (47, 50, 51), (12, 55, 65), along with other muscles (ECR, ECU, EDC, FCR, FCU, FDS, and quadriceps).
Depending on the mechanical/electrophysiological parameter of interest, the experimental setup equipment for recording/producing movement is also presented in Table 2. Since the amplitude and velocity of joint movement affect rigidity assessment (30), most studies reported these two values during their assessments. Two columns have been added to Table 2 to show the amplitude and velocity of the movement. However, some researchers failed to mention these effective values in their papers (11, 14, 19), (21, 27, 28), (32, 35, 37), (38, 40, 51), (58, 60, 65).
Significant findings from each study are presented in Table 3. These findings compare the BOMs of the study with clinical measures as the reference. Among the BOMs, the following were more frequently used for evaluating rigidity: Torque-angle integral (work) (9, 11, 14), (18-20), (22, 30, 32), (37, 42, 45), (46, 47, 49, 51), torque-angle slope (2, 18, 22), (23, 25, 30, 32), torque integral with respect to time (impedance) (12, 21, 25), viscoelastic parameters (10, 12, 15), (18, 24, 33, 44), and aspects of the EMG signal (2, 9, 13),(14, 19, 23), (30, 32, 37, 38, 44).
From clinical scales, only the UPDRS was used by 23 studies (7-9), (15, 17-19), (24, 25, 28), (29, 32, 34), (37, 39, 41), (44, 45, 47), (49, 56, 59), (55, 58), Hohen and Yahr by five studies (16, 36, 43, 64, 67), while 16 studies used both Hohen and Yahr and UPDRS (2, 11-13), (21-23), (27, 30, 31), (42, 50, 62), (60, 63). One study used the Webster Disability Grading (35, 51), and another used the Columbia University Rating Scale (40). These parameters are defined under experimental conditions, which often include velocity, amplitude, frequency, regression UPDRS index, medicine use, DBS treatments, movement direction (wrist flexion and extension), and joint state (active or inactive) (Table 3).
5. Discussion
Parkinson's disease affects 0 - 3% of individuals in industrialized nations and 1% of those aged over 60 (3, 4). A comprehensive study is needed to extract and compare objective rigidity (OR) measures over the past 40 years, categorized as CAT-1, CAT-2, and CAT-3, based on the MPs used.
5.1. Studies in CAT-1
Six studies exclusively used EMG (MP1) for rigidity assessment. In 1993, Mary and Cody identified an enhanced stretch reflex as a possible source of rigidity, supported by the torque needed for wrist flexion reaching 20% of its maximum (28). In another 1992 study, they examined rigidity in nine Parkinson's patients, recording rigidity with normalized EMG and displacement during flexion and extension (34), again suggesting the enhanced stretch reflex as the source of rigidity (28, 34).
Cantello et al. recorded EMG in resting FDI muscles and proposed that abnormal motor unit discharge at rest in rigid muscles is the primary cause of rigidity, overlooking viscoelastic factors (27). Bergui et al. recorded quadriceps muscle EMG and observed an enhanced delay in the stretch reflex in Parkinson's patients, contributing to rigidity (25). In 2015, Kwon et al. used EMG parameters to investigate the contribution of shortening reactions and stretching reflex in wrist muscle rigidity, concluding that shortened wrist muscles have higher EMG activity than stretched muscles, indicating unequal contributions to rigidity (44).
5.2. Studies in CAT-2
Twenty-four studies employed kinetic and kinematic parameters (MP2 - MP3) to assess rigidity in PD. These studies primarily focused on recording torque and angle in various joints (wrist, elbow, neck, trunk) (2, 7, 10), (11, 13, 14), (16, 18, 20), (21, 22, 24), (29, 32, 36), (40, 42, 43), (46, 49, 56), (61-63), (58, 60). Commonly extracted BOM parameters from these studies include work, impedance, angle of the torque diagram, viscoelastic components, and more (2, 7, 8), (9-11), (13-15), (16, 17, 19), (20-23), (25, 28, 29), (30-32), (34, 36, 37), (40-42), (43-45), (46, 47, 49), (50, 51, 56), (12, 59, 61) (62-64), (55, 58, 60, 65) (Table 3).
Researchers have evaluated these parameters under various medication conditions, movement speeds, frequencies, amplitudes, and other factors. For instance, Xia et al. (2, 22) examined the impact of rigidity on extension and flexion, finding a greater impact on extension and noting the efficacy of medication in controlling extension rigidity (31, 41, 64). Rigidity and akinesia progress more rapidly compared to tremors in Parkinson's patients, a finding confirmed by Robichaud and Endo et al. in 2004 and 2009 using other BOMs (22, 41, 45, 47, 68). Mak et al. (29) recorded trunk rigidity and suggested several BOMs, all showing high confidence in evaluating trunk rigidity while considering viscoelastic parameters. Teravainen et al. extracted a BOM based on wrist work, and their suggested range for viscoelastic parameters became a standard in experimental design for many researchers, even those using different BOMs (13, 16, 32), (7, 44, 49).
Park et al. conducted a study on kinetic-kinematic parameters (CAT_2), finding a higher correlation between BOMs in the first category and clinical scores compared to EMG-based measures (CAT_1). This superiority was supported by Kwon et al., who reported a strong correlation between viscoelastic parameters and the UPDRS index (7). Webster, in 1959, suggested devices for rigidity evaluation using a work-based BOM, which demonstrated acceptable sensitivity. In 2011, Park et al. confirmed this approach, showing a significant correlation between work-based and impulse-based BOMs and UPDRS scores (7). In 2015, a tool called BiRD was developed for Metacarpophalangeal rigidity registration, showing a moderate correlation with the MDS-UPDRS clinical score (42).
Costa et al. in 2015 created a wearable sensor for wrist rigidity assessment, developing a polynomial model using extracted BOMs. This model successfully detected rigidity in Parkinson's subjects compared to healthy controls, with clinical measures for rigidity provided by two blinded physicians (60). Additionally, biomechanical investigations have shown that OR in PD progressively increases with higher angular velocities during robot-assisted wrist extensions (24, 61).
5.3. Studies in CAT_3
Twenty-two studies used a combination of electrophysiological, kinetic, and kinematic measures (MP1-MP2-MP3) to evaluate rigidity in PD, introducing various successful BOMs. For instance, Xia and Powell introduced measures such as work, impedance, and EMG under different experimental conditions (8, 14, 15), (17, 19, 23),(30, 31, 35), (37, 38, 41),(45, 47, 50), (12, 59, 61), (55, 64-66) (Table 3).
In 2011, the effects of velocity and amplitude changes on rigidity were assessed using BOMs like work, impulse, torque angle slopes, and EMG-based measures. The impact of contralateral activation on rigidity was also evaluated using these BOMs (13, 15, 45).
Xia et al. in 2016 investigated the neural and mechanical origins of rigidity in PD, finding that individuals with Parkinson's have a higher stretch reflex and biomechanical component compared to control groups. Dopaminergic medication had a more significant effect on the neural component of rigidity (30, 59). Fung et al. in 2000 concluded that angular impulse correlates better with UPDRS scales compared to work as a BOM. Sepehri et al. in 2007 compared work-based BOMs with other biomechanical and physiological-based BOMs, demonstrating that work-based measures have a better correlation with clinical scores, corroborating earlier findings by Webster (19). In 2020, Ferreira-Sánchez et al. conducted a meta-analysis assessing the reliability of 36 papers that evaluated rigidity and stiffness in Parkinsonian patients' muscles. These studies reported that all the methodologies presented in these papers demonstrated excellent validity and reliability, exhibiting robust correlations with clinical assessment scales (26).
The aim of this study, distinct from the aforementioned ones, is to derive and classify biomechanical BOMs for recording rigidity in Parkinson's patients, with the hope of establishing a relationship and equation between objective and subjective parameters.
5.4. Conclusions
Based on the analysis of 53 studies, critical biomechanical measures for assessing rigidity in Parkinson's patients include viscoelastic parameters, EMG signals, impedance, work, torque-angle slopes (stiffness), and impulse. These measures show a strong correlation with UPDRS scores. Classifying and comparing them may help establish precise predictive models for rigidity stages under different conditions. These models could aid in monitoring the progression of rigidity and akinesia, which is faster than tremors. They may also enhance the effectiveness of interventions like deep brain stimulation. Furthermore, these predictors could promote better communication between biomechanists and neurologists when discussing rigidity in PD.