1. Background
Leishmaniasis is a widespread parasitic disease affecting humans and animals, transmitted by sand fly mosquitoes. Although observed globally, 90% of cases are concentrated in eight countries: Iran, Afghanistan, Syria, Iraq, Saudi Arabia, Algeria, Brazil, and Peru (1). The World Health Organization (WHO) considers it a major health concern. First-line treatments primarily rely on pentavalent antimony compounds, with Amphotericin B and Pentamidine used in advanced cases. Currently, there is no effective vaccine against leishmaniasis, highlighting the need for new drugs to replace or complement existing therapies (2, 3).
Pentavalent antimonials have significant side effects, including pancreatitis, hepatitis, bone marrow suppression, and electrocardiographic changes. Similarly, Pentamidine is associated with adverse effects such as diabetes mellitus, renal toxicity, and anemia. Amphotericin B, an antifungal agent active against Leishmania species, also presents a high incidence of side effects (4, 5). When secondary bacterial and fungal infections complicate lesions caused by the parasite, these infections can exacerbate the condition, sometimes surpassing the primary leishmaniasis infection in severity. Consequently, in endemic areas, wounds are often treated with drugs that may have unexpected side effects (6, 7). Currently, no specific guidelines exist on the timing or application of antibiotics as adjunctive therapy for cutaneous leishmaniasis (CL), and they are typically prescribed for cases with pain and inflammation (8, 9).
Scrophulariabavanatia predominantly grows in mountainous regions and is part of Iran’s native flora. It possesses therapeutic properties, functioning as a cardiac stimulant, blood circulation enhancer, diuretic, antipyretic, antibacterial, anti-inflammatory, and wound healing agent. Due to the medicinal potential of Scrophulariabavanatia extract (SBE), this study aimed to investigate its antileishmanial effect on L. tropica parasites (10).
2. Objectives
This study assessed the in vitro antileishmanial effect of local Scrophulariabavanatia extract on Leishmaniatropica [MHOM/NADIM3] promastigotes.
3. Methods
3.1. Preparation of Herbal Extract
In this laboratory experiment, the aerial parts of Scrophulariabavanatia were first dried in an oven at 30°C. The dried material was then soaked in 80% methanol for seven days. Following this, the solvent was evaporated, and the extract was lyophilized. The resulting extract was dissolved in Dimethyl Sulfoxide (DMSO, BDH, England) at a concentration of 20 mg/mL and stored at 4°C for subsequent use (11).
3.2. Parasite Culture
The Iranian standard strain of L. tropica (MHOM/NADIM3) promastigotes was stored at the Department of Parasitology and Mycology, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. L. tropica promastigotes [MHOM/IR/NADIM3] were cultured in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin, and 100 IU/mL penicillin at 26°C. Promastigotes (1 × 106 parasites/mL) were incubated at 26°C for 24, 48, and 72 hours in fresh medium, either without or with various concentrations (0.2, 1, 5, 25, 125 mg/mL) of the methanolic extract of Scrophularia bavanatia. To ensure data validity and reliability, concentrations ranged from the lowest (0.2 mg/mL) to the highest (125 mg/mL) and were tested in triplicate. Promastigotes in the stationary growth phase were added to the wells of the plate. A positive control group (Glucantime: 25 and 125 μg/mL) was also included (12, 13). The anti-leishmanial activity of the Scrophulariabavanatia extract was assessed by comparing results from the positive control (Glucantime: 25 and 125 μg/mL) and the experimental groups, designed in alignment with all laboratory test protocols as follows:
Group 1: 100 µL of RPMI1640 + 1 × 106 cells/mL PMs + 10 µL of 0.2 hydro alcoholic SBE (Case)
Group 2: 100 µL of RPMI1640 + 1 × 106 cells/mL PMs + 10 µL of 1 hydro alcoholic SBE (Case)
Group 3: 100 µL of RPMI1640 + 1 × 106 cells/mL PMs + 10 µL of 5 hydro alcoholic SBE (Case)
Group 4: 100 µL of RPMI1640 + 1 × 106 cells/mL PMs + 10 µL of 25 hydro alcoholic SBE (Case)
Group 5: 100 µL of RPMI1640 + 1 × 106 cells/mL PMs + 10 µL of 125 hydro alcoholic SBE (Case)
Group 6: 100 µL of RPMI1640 + 1 × 106 cells/mL PMs + 10 µL of 25 µg Glucantime (Positive control)
3.3. Cell Proliferation ELISA, Brdu (Chemiluminescent) Method
The XTT detection kit (Sigma, St. Louis, MO, USA) was used for the anti-leishmanial bioassay employing the chemiluminescence method. XTT solution was prepared at a concentration of 5 mg/ml in RPMI1640 medium without phenol red, and 20 μL of this solution was added to each well, followed by incubation at 25°C for 24 hours. Following incubation, 150 μL of acidic isopropanol was added to each well to dissolve the formazan crystals. The absorbance was measured using an ELISA reader at a test wavelength of 450 nm with a reference wavelength of 630 nm (14).
3.4. Statistical Analysis
The data were analyzed using SPSS 26 (IBM Inc., Chicago, IL, USA). All experiments were conducted in triplicate, and the mean and standard error were calculated from at least three independent experiments. Statistical analysis of differences between mean values across experimental groups was performed using Student’s t-test, with significance set at P ≤ 0.05.
4. Results
The results of examining the LC50 of Glucantime and the hydroalcoholic extract of Scrophulariabavanatia against promastigotes in the dynamic and stationary stages of Leishmaniatropica [MHOM/IR/NADIM3] are reported. As expected, the LC50 values in the stationary stage for both the hydroalcoholic extract of Scrophulariabavanatia and Glucantime (mg/mL) were higher than in the dynamic stage. The effectiveness of Glucantime and the hydroalcoholic extract of Scrophulariabavanatia on the survival of Leishmania promastigotes at concentrations of 0.2, 1, 5, 25, and 125 mg/mL after 24, 48, and 72 hours is shown in Table 1.
| Promastigote Type | Hydro Alcoholic Extract of SB (mg/mL) | Glucantime (mg/mL) |
|---|---|---|
| Dynamic stage | 47.1 | 368.2 |
| Stationary stage | 53.2 | 377.6 |
The Mean LD50 of Glucantime and the Hydro Alcoholic Extract of Scrophulariabavanatia Extract Against Promastigotes of the Parasite L. tropica [MHOM/IR/NADIM3]
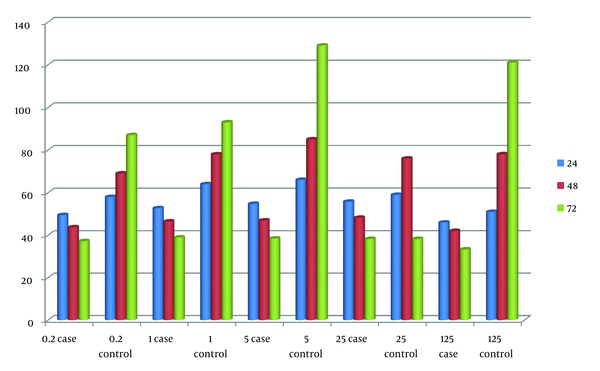
The repeated measures test demonstrated that Glucantime (positive control) showed no significant difference at 24 and 48 hours compared to the positive control group; however, it displayed a significant difference at 72 hours, as indicated in Table 2. Similarly, the repeated measures test suggested that the hydroalcoholic extract of Scrophulariabavanatia did not show any significant difference at 24 and 48 hours compared to the positive control group, but it exhibited a significant difference at 72 hours, as shown in Figure 1.
| Concentration Hour | 0.2 | 1 | 5 | 25 | 125 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SBE | Control | SBE | Control | SBE | Control | SBE | Control | SBE | Control | |
| 24 | 49.500 ± 4.65 | 58.00 | 52.75 ± 6.39 | 64.00 | 54.75 ± 4.34 | 66.00 | 55.75 ± 4.50 | 59.00 | 46 ± 4.43 | 51 |
| 48 | 43.75 ± 5.31 | 69.00 | 46.500 ± 6.35 | 78.00 | 47.00 ± 5.35 | 85.00 | 48.25 ± 4.64 | 76.00 | 42 ± 4.31 | 78 |
| 72 | 37.25 ± 4.99 | 87.00 | 39.00 ± 6.05 | 93.00 | 38.50 ± 5.91 | 129.00 | 38.25 ± 3.59 | 93.00 | 33.31 ± 2.20 | 121 |
Evaluation of the Effect of Glucantime (Positive Control) on the Survival of Promastigotes of the Parasite L. tropica in 0.2, 1, 5, 25, 125 Concentrations of Scrophulariabavanatia Extract (Cases) and Control after 24, 48, and 72 h a
5. Discussion
As anticipated, the LC50 of the stationary phase for both the hydroalcoholic extract of Scrophulariabavanati and Glucantime (in mg/mL) was higher than in the dynamic phase. Glucantime did not show a significant difference at 24 and 48 hours compared to the experimental groups; however, a significant difference was observed at 72 hours. Similarly, the hydroalcoholic extract of Scrophulariabavanati did not display a significant difference at 24 and 48 hours compared to the positive control group but demonstrated a significant effect at 72 hours.
The primary treatment for Leishmaniasis currently relies on tri- and pentavalent antimony compounds such as Glucantime and Pentostam, which require prolonged administration and can have severe effects on the kidneys and liver. Additionally, the use of Glucantime is often painful and can cause joint stiffness, making it difficult for many patients to tolerate. Consequently, there is still no universally effective or suitable medication for Leishmaniasis (9, 15). Numerous studies have explored the use of various herbal extracts to treat Leishmania, with each extract showing potential benefits for different symptoms of the disease. In some cases, these extracts have reduced inflammation, slowed the progression of ulcers, and prevented secondary infections.
Cutaneous Leishmaniasis, caused by a flagellated protozoan of the genus Leishmania, is transmitted to humans through sand fly bites, typically resulting in small nodules that develop into slow-healing wounds. Lesions often appear on exposed areas of the body, such as the hands, feet, legs, and face. In Iran, Cutaneous Leishmaniasis is a significant health issue, with up to 26,000 cases reported annually. This disease is prevalent among both humans and animals, especially in open areas. The cities of Tehran, Shiraz, Mashhad, Kerman, Neyshaboor, Isfahan, and Yazd are among the primary centers for L. tropica in Iran. Additionally, significant centers for Leishmaniamajor in Iran and Turkmenistan include the districts of Sarakhs, Lotf Abad, Turcoman Sahara, and Esfarāyen in Khorasan, as well as Khuzestan, Qom, Kashan, Yazd, and Tabas. Specifically, important foci in Isfahan are located in Barkhar, Meimeh, Ardestan, Natanz, and ‘Aran and Bidgol’ (16, 17). The calculated LC50 concentration, at which 50% of the pathogenic parasite is eliminated, showed that the LC50 of the hydroalcoholic extract of Scrophulariabavanatia is significantly lower than that of the current standard treatment, Glucantime (positive control). Secondly, as anticipated, the LC50 level for promastigotes in the dynamic stage of L. tropica is lower than in the stationary stage, as the dynamic stage is non-infectious and non-pathogenic for the vertebrate host; however, the stationary stage is both infectious and pathogenic for the vertebrate host (humans) (17, 18). Exposure of L. tropica to the hydroalcoholic extract of Scrophulariabavanatia at concentrations of 0.2, 1, 5, 25, and 125 mg/mL for 24 hours did not show any significant difference compared to the positive control group, indicating that prolonged exposure to the herbal extract is necessary for the parasites to be affected. The effectiveness of herbal medicines in alleviating the symptoms of Leishmaniasis has been demonstrated in numerous studies on medicinal plants and Leishmania. Fattahi et al. investigated the effect of Onosmastenosiphon extract on the Leishmania parasite (9). Similarly, Mirzaei examined the impact of Satureja hortensis and Artemisiadracunculus extracts on Leishmania parasites (8). However, exposure of the L. tropica parasite to the hydroalcoholic extract of Scrophulariabavanatia at various concentrations (0.2, 1, 5, 25, and 125 mg/mL) for 48 hours did not show any significant difference compared to the positive control group. This suggests that the Leishmania parasite requires at least 48 hours of exposure to the hydroalcoholic extract to observe any effect (19, 20). Additionally, it is evident that L. tropica [MHOM/IR/NADIM3] is more resistant and requires a longer duration and higher dosage of treatment compared to L. major [MRHO/IR/75/ER]. When the L. tropica parasite was exposed to the hydroalcoholic extract of Scrophularia bavanatia at concentrations of 0.2, 1, 5, 25, and 125 mg/mL for 72 hours, a significant effect was observed compared to the positive control group. This suggests that a 72-hour exposure is required for the extract to be effective against L.tropica. Similarly, when the L. tropica parasite was exposed to Glucantime (positive control) at the same concentrations for 24 hours, no significant effect was noted compared to the negative control group, indicating that a longer exposure is also needed for Glucantime to be effective. Additionally, exposure to Glucantime for 48 hours showed no significant difference compared to the positive control group, reinforcing the need for a minimum of 48 hours of exposure for effectiveness.
This data highlights that L. tropica [MHOM/IR/NADIM3] is more resistant and requires longer exposure and higher dosages than L. major [MRHO/IR/75/ER] for effective treatment, even with pentavalent antimony compounds like Glucantime. However, Glucantime is ultimately capable of eradicating both L. major and L. tropica within 72 hours. Finally, various factors such as environmental conditions (climate, parasite reservoirs), human and animal population density, parasite strain (which affects pathogenicity), and host-related factors (age, sex, immune status, nutrition) also contribute to treatment outcomes.
5.1. Conclusions
The primary challenge in treating leishmaniasis is selecting drugs that are both less complex and less toxic while exhibiting high sensitivity against the parasite. Therefore, the need for an effective alternative to chemical drugs is more urgent than ever. These results indicate that Scrophulariabavanatia possesses medical potential comparable to Glucantime, with a more pronounced impact on the survival of promastigotes, minimal risk of parasite resistance, no complications, and broader availability. However, further research should explore advanced formulations, such as nanoparticle-based extracts, to evaluate efficacy in cell cultures and in vivo conditions.
Based on the findings of this study, we conclude that numerous medicinal derivatives and herbal extracts demonstrate relatively strong antileishmanial activity compared to synthetic compounds. Given that plant-based compounds are generally safer, more affordable, and simpler to produce than Glucantime, their potential use in the treatment of leishmaniasis is a promising avenue for future treatment strategies.