1. Background
Water has a major role in the environment and subsequently in human life. The population increment followed by industrial and agricultural development have led to generation of large amounts of waste water. Industries are one of the major sources of environmental pollution and heavy metals are one of them. Agricultural pollution occurs due to using fertilizers which consist of heavy metals. Heavy metals are hazardous for human life because of their intake and bioaccumulation in human bodies through food chain (1). The common way of exposure to lead (Pb) is food and edible water. Pb-based dyes, aerosols, combustion gases of fossil fuel, and battery manufacturing industries are other potential sources of exposure to Pb (2). Cadmium (Cd) intake can occur via food and edible water, too. Phosphate fertilizers are the major sources of Cd pollution. Aerosols, dyes, Ni-Cd battery manufacturing industries, heating process systems like iron, and cement production are other sources of Cd environmental pollution (2).
To date, various methods have been applied for the removal of heavy metals; the most commonly used of them are ion exchange (3), reverse osmosis (4), membrane filtration (5), and solid phase extraction (SPE) (6). In recent years, SPE has become an interesting solution for removal of heavy metals from water and wastewater, owing to the capability of this method to adsorb heavy metal ions rapidly, efficiently, inexpensively and adaptive to environment, compared to other methods. Nowadays, nanotechnology has become one of the most efficient solutions for wastewater treatment in the world. This new technology has been an advantageous replacement of the traditional practices for water treatment (7).
Magnetic zero-valent iron nanoparticles (ZVINPs) are of SPE methods which are more attractive in comparison to other methods of SPE such as activated carbon (8), fly ash (9), lignin (10), and zeolite (11), due to their simplicity and ease of operation. Today, removal of heavy metals, especially Pb and Cd has become a subject of debate in environmental organizations. The investigated method shows satisfactory efficiency to remove at even higher concentration from water in complicated media like sea water. The effects of empirical parameters such as pH, contact time and adsorbent amount have been optimized. in addition, the capability of ZVINPs as efficient extractors has been studied for preconcentration and determination of Pb and Cd in water samples.
2. Objectives
The aim of this study was to investigate the adsorption properties of ZVINPs with Pb(II) and Cd(II). Moreover, feasibility of the adsorbent to extract Pb and Cd ions was utilized to preconcentrate and determine the desired ions. The effects of factors such as pH, contact time, amount of ZVINPs, electrolyte, volume of sample solution, type of acidic digestive solution, and volume of digestive solution, were studied.
3. Materials and Methods
3.1. Instrumental
An AA240FS atomic absorption spectrophotometer (Varian, Australia) equipped with Pb and Cd hallow cathode lamps (Photron, Australia) at 283.3 nm and 228.8 nm, was used for determination of Pb and Cd in sample solutions, respectively. As necessary, flame or electrothermal system was used. Considering the range of analyte concentration, flame atomic absorption (FAAS) (at mg/L range) and graphite furnace atomic absorption (GFAAS) (at µg/L range) were used. Transmission electron microscopy (TEM) was performed using a field emission TEM (906E, LEO, Germany), operating at 200 kV to measure the particles size and shape. Structural analysis of ZVINPs was carried out using X-ray diffractometer (XRD, Brucker D8 Discover, Germany). An IKA Works KS 130 Orbital Shaker (IKA Works Basic Model), a Jenway hotplate and stirrer (model 1000, UK), a Metrohm pH meter (model 827, Switzerland) and a magnet (1.2 T, 10 × 5 × 2 cm) were used during the experiments.
3.2. Chemicals
All the solutions were prepared by double-distilled deionized water with electric conductivity below 1.5 µS/cm. All the chemicals and reagents were of the analytical grade. FeCl3 anhydrous powder (≥ 99.99%, w/w) was purchased from Sigma-Aldrich (Switzerland). NaBH4, Suprapur® nitric acid (65%, w/w), and Suprapur sulphuric acid (96%, w/w) were purchased from Merck (Darmstadt, Germany). Analytical solutions of Pb and Cd were prepared by step-by-step dilution of 1000 mg/L standard solutions (Romil, UK) with water.
3.3. Preparation of Zero-Valent Iron Nanoparticles
ZVINPs were prepared, retrieved from the method studied by Sun et al. (12). Briefly, ZVINPs particles were synthesized by reduction of ferric chloride with sodium borohydride under N2 atmosphere. For synthesis of 100 mg ZVINPs, 1.8 mL of 1 M Fe3+ solution was added to 200 mL water, and 10 mL NaBH4 (1 M) was slowly added in one minute during mixing the solution by a magnetic stirrer at 600 rpm. Reduction of Fe3+ was performed according to the following reaction:
4 Fe3+ (aq) +3 BH4−(aq) + 9 H2O → 4 Fe0 + 3 H2BO3−(aq) + 12 H+(aq) + 6 H2(g)
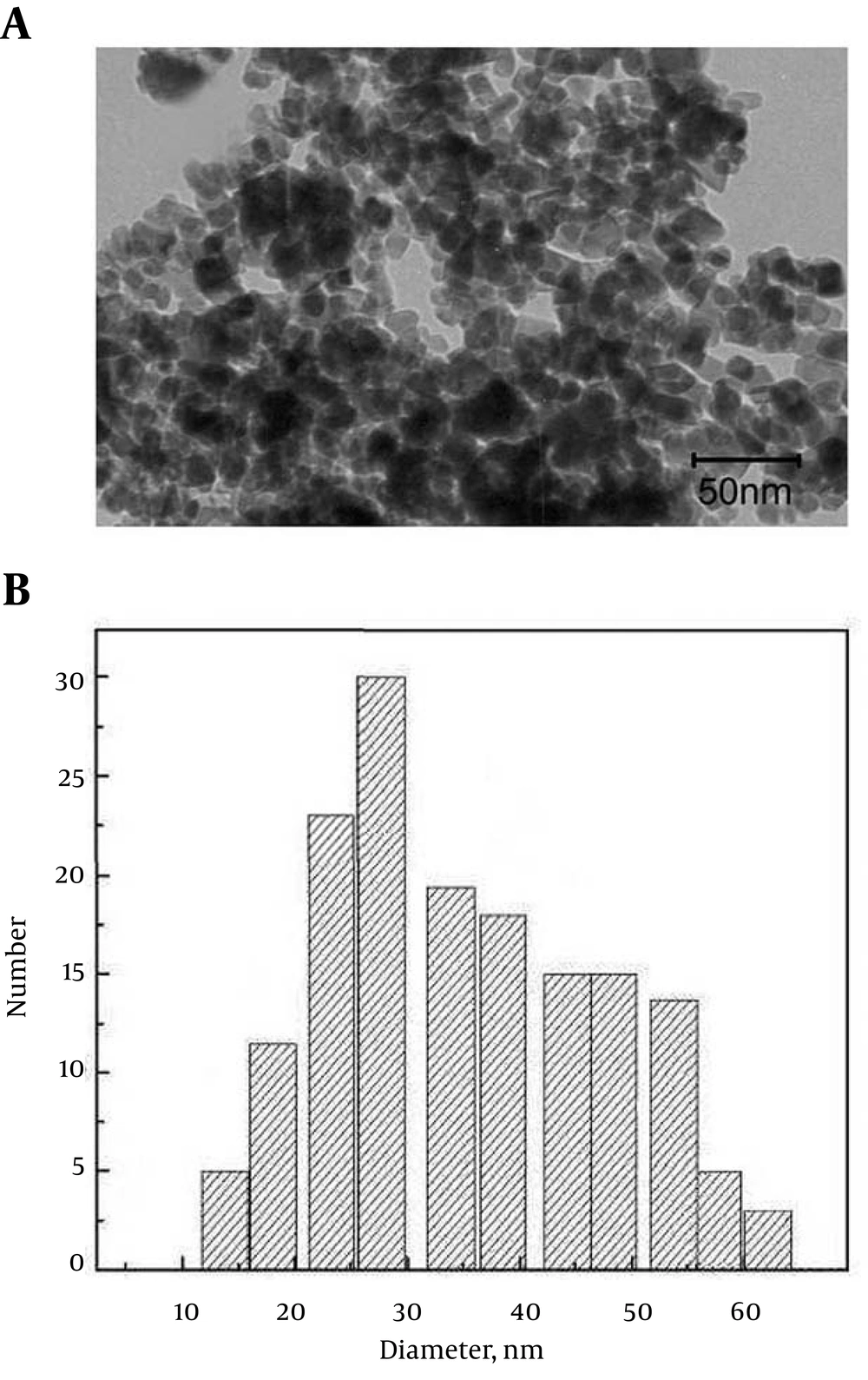
The synthesized ZVINPs were settled at the bottom of a beaker using a strong magnet and washed with distilled water a few times until the pH of the washing solution reached 7. Figure 1 shows the TEM image and the graph of size distribution of the synthesized ZVINPs (the average sizes of quasi-spherical ZVINPs are about 20 - 50 nm).
3.4. Adsorption Procedure
The adsorption behaviors of Pb(II) and Cd(II) onto ZVINPs were studied by batch adsorption experiments on an orbital shaker at 300 rpm, using 250 mL conical flasks. In a typical experiment, 40 mg of ZVINPs was added to 50 mL of mixed Pb (5 mg/L) and Cd (1 mg/L) solution at 25°C with pH = 7. After two minutes (the required contact time for almost completing the adsorption of Pb and Cd ions), the ZVINPs were separated from the solution by a magnet, as an external magnetic field.
3.5. Preconcentration and Determination
The experimented method was studied by dissolving Pb- and Cd-loaded ZVINPs with an acidic digestive mixture solution of HNO3 and H2SO4 (4:1 HNO3 65% and H2SO4 98%, respectively). Pb and Cd of the test solution (100 mL) were adsorbed according to the procedure described above. After settlement of the adsorbent and removing the supernatant of the test solution, 5 mL of the digestive mixture was added to ZVINPs. The acidic mixture dissolves Pb- and Cd-loaded ZVINPs in about 30 minutes. After dissolving the adsorbents, the acidic solution was ready to be introduced to FAAS. Dissolved non-Pb and Cd-loaded ZVINPs were introduced to FAAS as a blank sample prior to analyzing the Pb and Cd ions in the sample solutions.
3.6. Adsorption Study
The adsorption experiments of Cd2+ and Pb2+ were derived at batch mode; 40 mg of the adsorbent was added to 50 mL of the metal ion solution in 250 mL conical flasks at controlled conditions, including the initial pH ranging 6 - 8 and stable room temperature (25 ± 0.1°C). The speed of stirring the solution was fixed at 300 rpm by a magnetic stirrer. After 30 minutes of stirring, the ZVINPs were separated from the solutions using an external magnetic field and the initial and final metal ion concentrations were determined by FAAS.
3.7. Sampling
The proposed method was applied to different water samples, including 1) Well water (Shushtar, Iran), 2) Karoon River (Ahvaz, Iran), 3) Caspian Sea (northern Iran), and 4) Persian Gulf (southern Iran). All the samples were filtered through a 0.45 µm cellulose acetate membrane filter and preserved at pH = 2 with 0.1 M HNO3. Before analysis, pH of the sample solution was adjusted at 7 ± 1 by 0.1 M NaOH. Adsorption, preconcentration and determination processes were performed on the samples according to the procedure described in this study.
4. Results
4.1. Characterization of Zero-Valent Iron Nanoparticles
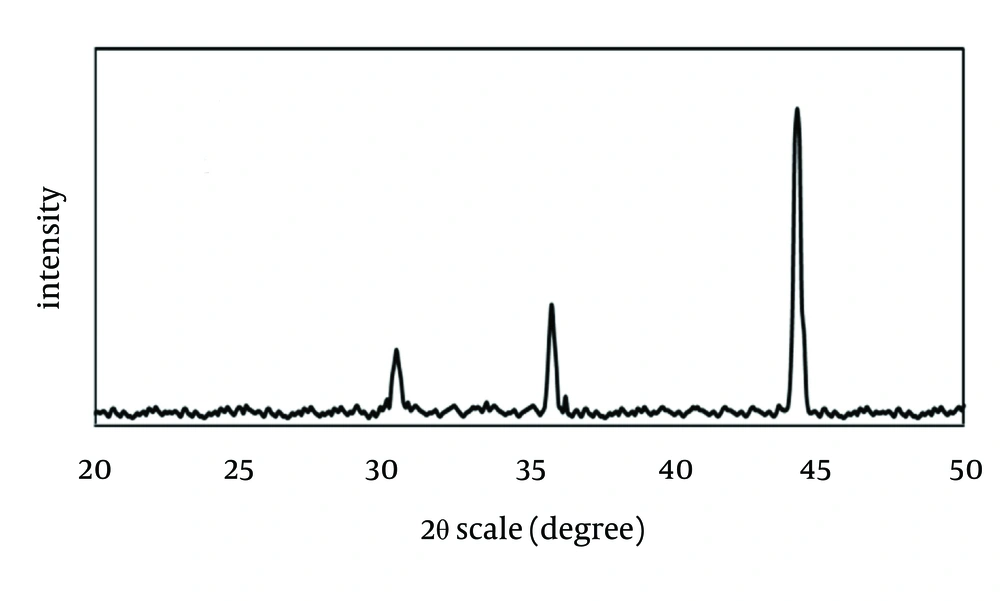
The X-ray diffraction (XRD) spectrum of synthesized ZVINPs is presented in Figure 2. It can be concluded that the peak at 2θ = 44.34° is due to the body-centered-cubic (bcc) phase of Fe (α-Fe). Some FeO (due to oxidation of Fe) diffraction peaks of iron oxides can be observed in the spectra.
4.2. Effect of pH
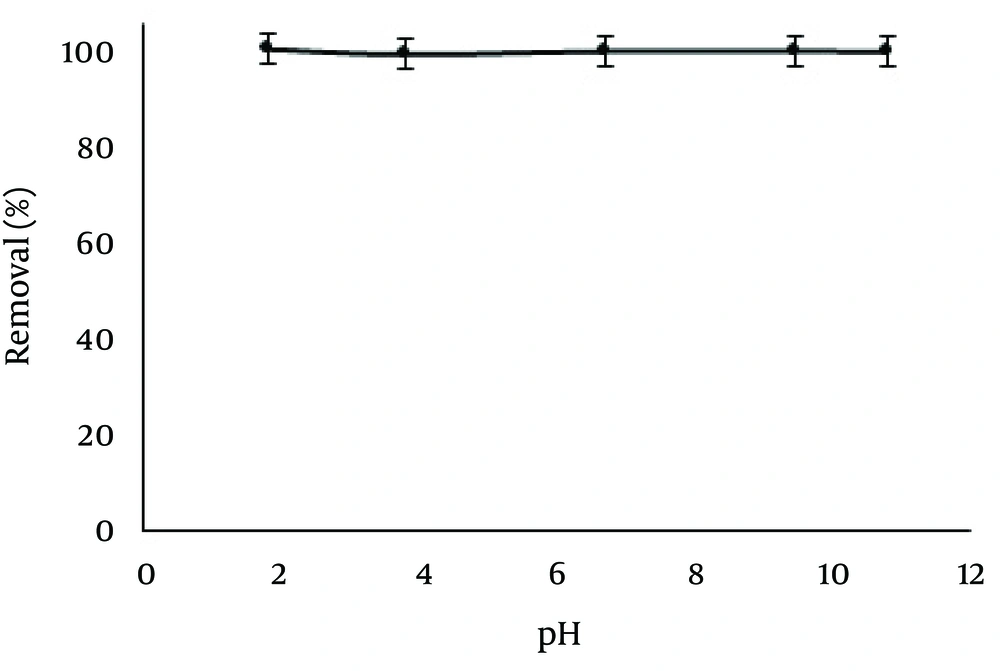
The effects of pH on the adsorption of Pb and Cd ions by ZVINPs were investigated at pH of 2.0 - 8.5 (50 mL, Pb2+ 5.0 and Cd2+ 1.0 mg/L, 25°C, contact time: 30 minutes). Since Pb(II) and Cd(II) precipitate with OH- at the basic pH and on the other hand ZVINPs are dissolved at pH < 2, control of pH seems to be serious during the analysis. Generally, bare ZVINPs can adsorb heavy metals on their surfaces through different mechanisms. The adsorption mechanism of Pb(II) and Cd(II) on ZVINPs can be described by the standard redox potential (E0). Metals that have an E0 which slightly more positive than Fe0 (like Pb and Cd), can be removed by reduction or precipitation mechanisms on iron oxide shell around ZVINPs (13). Previous studies showed that removal of Pb(II) from aqueous solutions occurs through a physical mechanism (precipitation) (14), while the chemical is more preferable for Cd(II) adsorption due to reduction on ZVINPs surface (15). As shown in Figure 3, in the studied concentrations of the desired ions, pH did not have any significant effect on Pb and Cd adsorption mechanisms.
4.3. Effect of Contact Time
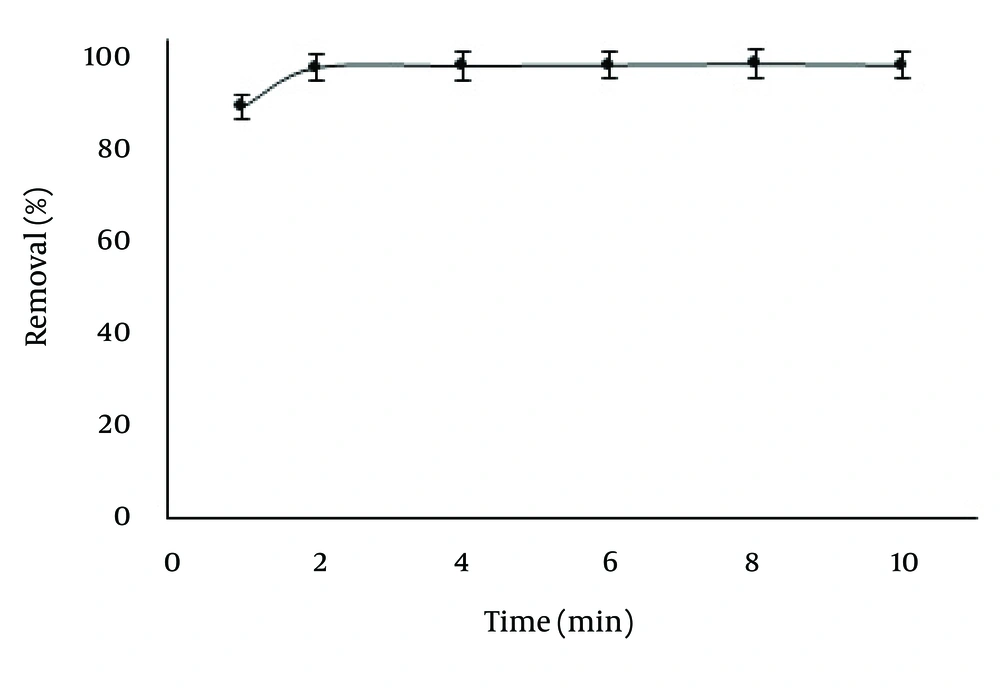
The effect of contact time on the adsorption of Pb(II) and Cd(II) was studied at different intervals in the range of 1 - 10 minutes. (mixture of Pb2+ 5.0 mg/L and Cd2+ 1.0 mg/L, 50 mL, pH = 7.0, 25°C). After the settlement of ZVINPs by a magnet for about one minute, the supernatant solutions were collected for analysis of heavy metal ions using GFAAS. The optimum contact time was two minutes for both Pb and Cd, even at the presence of each other in the solution (Figure 4). Increasing the contact time between contaminated water and adsorbent can decrease the level of Pb and Cd to the permitted healthy levels recommended by World Health Organization (WHO) (16).
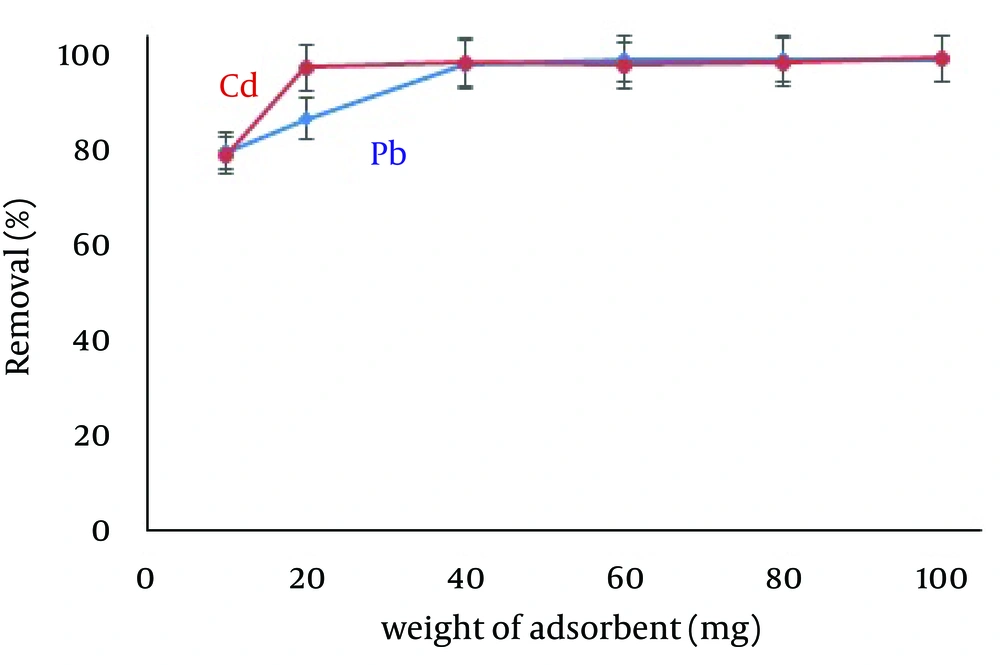
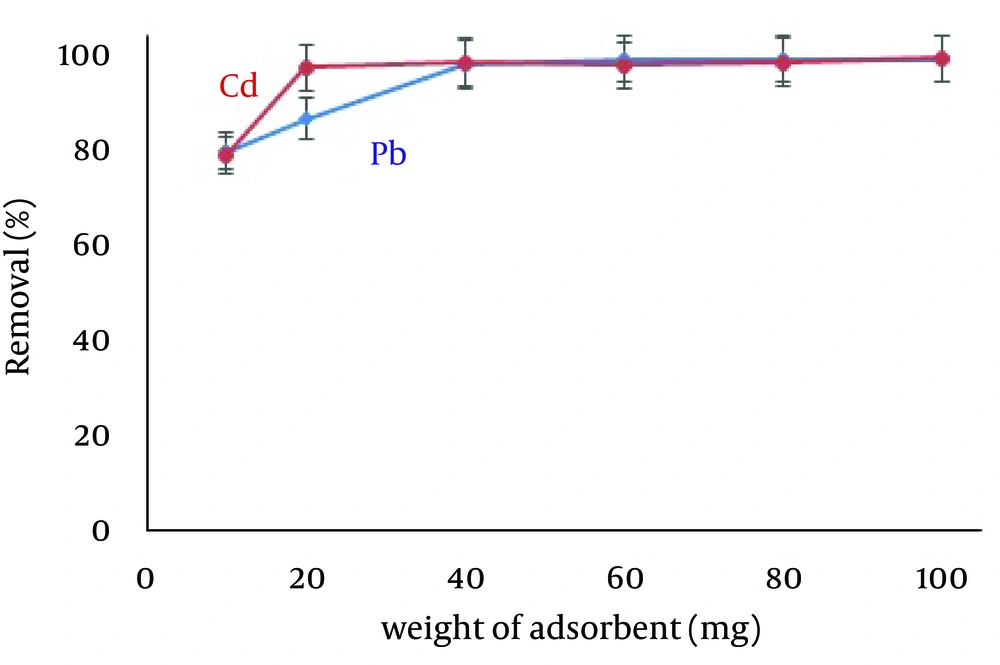
4.4. Effect of the Amount of Zero-Valent Iron Nanoparticles
The desired amounts of adsorbent required for adsorption of Pb (5.0 mg/L) and Cd (1.0 mg/L) from 50 mL solutions were obtained by studying the effects of different amounts of ZVINPs ranging from 10 to 100 mg. As shown in Figure 5, the operational amount of adsorbent intended for mixed solutions of Pb and Cd was 40 mg (removal > 95% with relative standard deviation relative standard deviation (RSD) below 3%). Maximum quantitative adsorptions were obtained while using 100 mg of ZVINPs (99.88% and 99.87% for Pb and Cd ions, respectively). Increasing the amount of ZVINPs enhanced the probability of collisions between adsorbent and metal ions in the solution.
4.5. Adsorption Isotherm
To determine the adsorptivity properties of Pb(II) and Cd(II) on ZVINPs, Langmuir and Freundlich adsorption isotherms were investigated. Langmuir isotherm refers to non-interactive monolayer adsorption of ions on a homogenous surface of adsorbent. Freundlich isotherm comes from multilayer adsorption of metal ions on heterogeneous surfaces. The capacity of ZVINPs for adsorption of Pb and Cd ions was achieved by measuring the initial and final concentration of the desired ions in the solution in a batch system (pH = 7.0, 25°C, stirring speed = 300 rpm and contact time 60 = minutes). Respectively, different concentrations of Pb2+ and Cd2+ ranging from 10 - 100 and 5 - 50 mg/L in water samples were examined with a fixed amount of adsorbent (40 mg). Both Langmuir and Freundlich isotherms were computed to realize the adsorption behaviors of Pb2+ and Cd2+ on ZVINPs adsorbents. As discussed, the results were collected before and after the removal process with respect to the calibration curve determined by the AAS system. The results showed that Langmuir model (r = 0.9977) fitted better than Freundlich model (r = 0.9713) for Pb(II) adsorption. In addition, the adsorption isotherm analyses for Cd(II) were deduced and Langmuir model (r = 0.9525) better fitted than Freundlich model (r = 0.9284), demonstrating that the adsorption of Pb(II) and Cd(II) on ZVINPs can occur through a monolayer process (Figure 6).
The Langmuir isotherm equation (Equation 1) was applied for explaining the correlation of the amount of metal ions adsorbed and its equilibrium concentration in solutions.

C (mg/L) is the equilibrium concentration of the metal ions in the solution, q (milligram of metal ions per gram of adsorbent) is the equilibrium adsorption amount of metal ions, qm is the maximum adsorption amount of metal ions per milligram of adsorbent (mg/g), and K is the Langmuir adsorption equilibrium constant in liter per milligram of adsorbent (L/mg).
The linear relationship between C/q and C (C/q = 0.0102 C + 0.0078 and C/q = 0.0323 + 0.0462 for Pb and Cd ions, respectively) showed the applicability of Langmuir model for the desired ions adsorption on ZVINPs. The experiments resulted in 1.31 and 0.69 for K and 98.03 and 30.95 for qm of Pb and Cd ions, respectively.
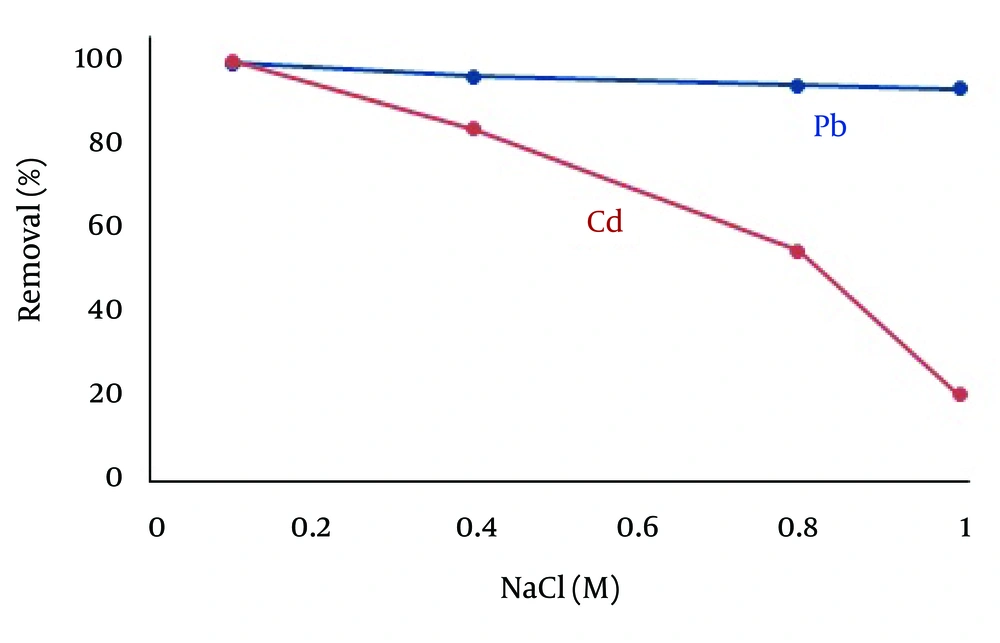
4.6. Effect of Electrolyte
In real situations, some salts may be present in water samples besides heavy metal ions like Pb and Cd and may interfere with heavy metal ions uptake by an adsorbent. To study the effect of ionic strength (adjusted by NaCl) on Pb and Cd adsorption, 50 mL of water sample (Pb2+ 5.0 mg/L, Cd2+ 1.0 mg/L) at 25ºC and pH = 7.0 was studied in the range of 0.01-1.00 M NaCl. It was observed that quantitative adsorption of Pb and Cd remained constant up to 0.1 M NaCl (removal efficiency was more than 99%). As shown in Figure 7, the influence of electrolyte on Cd2+ adsorption was more than Pb2+. Since the ion radius of Cd2+ is smaller than Pb2+, the charge density of Cd2+ can be greater than Pb2+. Increment of the charge density of an ion enhances the counter-ion layer (Cl-) around it. Increment of the counter-ion layer disturbs the interaction between metal ions and the negative surface of ZVINPs. Hence, the effect of electrolyte on Cd(II) adsorption is more than Pb(II).
4.7. The Effect of Solution Volume on Removal Process
Investigating the maximum operational solution volume was performed by fixing the total weight of Pb and Cd at 0.25 and 0.05 mg, respectively, while the dilution of the sample solution was increased. Different sample volumes between 25 and 200 mL were prepared (25°C, stirring speed = 300 rpm, contact time = 30 minutes, settlement time = 10 minutes, pH = 7.0). The obtained results showed that the maximum tolerable volumes for quantitative adsorptions of Pb and Cd ions were 100 and 75 mL, respectively (removal > 95%). At higher volumes metal ions were not effectively adsorbed, which was probably due to the lower feasibility of interaction between the adsorbate and adsorbent and decreasing the magnetic field strength at higher dilutions (more dilution causes increasing the volume, following decreasing the strength of magnetic field toward farther points of solution).
4.8. Loading Capacity
The loading capacity of adsorbent was determined under controlled conditions (pH = 7.0, 25°C, contact time = 30 minutes, stirring speed = 300 rpm) by batch method; 100 mg of the adsorbent was added to 50 mL of the solution, containing 100 mg/L of Pb and Cd. The removal percentage and the adsorbed amount of Pb and Cd were determined by flame and graphite furnace atomic absorption measurement of the sample solution before and after the removing process. The loading capacity was determined for Pb and Cd as 96.5 and 58.3 mg/g, respectively.
4.9. Study of Type and Composition of Acidic Digestive Solution
To preconcentrate and determine Pb and Cd ions in the aqueous solutions, ZVINPs were digested by the acidic solution after the adsorption process; 100 mL of the sample solution (Pb(II) 0.1 mg/L, Cd(II) 0.05 mg/L) was mixed with 40 mg adsorbent (pH = 7, 25°C, contact time = two minutes, stirring rate = 300 rpm). After the adsorption process, ZVINPs were separated by a magnet. The elapsed time for digestion of settled ZVINPs by a mixture of some acids was 30 minutes. To find out the best acid and/or composition of some, 4 mL of different acids like HNO3 65%, H2SO4 98%, HCl 37%, HClO4 60%, and their mixture were studied. According to the results, the best recovery was obtained when a mixture of nitric acid:sulfuric acid (4:1) was used (Table 1).
| Digestive Solution (Total Vol: 4.0 mL) | Recovery, % | |
|---|---|---|
| Pb | Cd | |
| H2SO4 (2.0 mL), HCl (2.0 mL) | 43 | 33 |
| HCl (2.0 mL), HNO3 (2.0 mL) | 52 | 43 |
| HClO4 (2.0 mL), HNO3 (2.0 mL) | 49 | 39 |
| HNO3 (4.0 mL) | 58 | 43 |
| HNO3 (3.5 mL), H2SO4 (0.5 mL) | 63 | 55 |
| HNO3 (3.2 mL), H2SO4 (0.8 mL) | 78 | 69 |
| HNO3 (3.0 mL), H2SO4 (1.0 mL) | 76 | 66 |
| HNO3 (2.0 mL), H2SO4 (2.0 mL) | 71 | 64 |
| HNO3 (1.5 mL), H2SO4 (2.5 mL) | 66 | 59 |
Study on the Type and Composition of Digestive Solution on the Recovery Method a
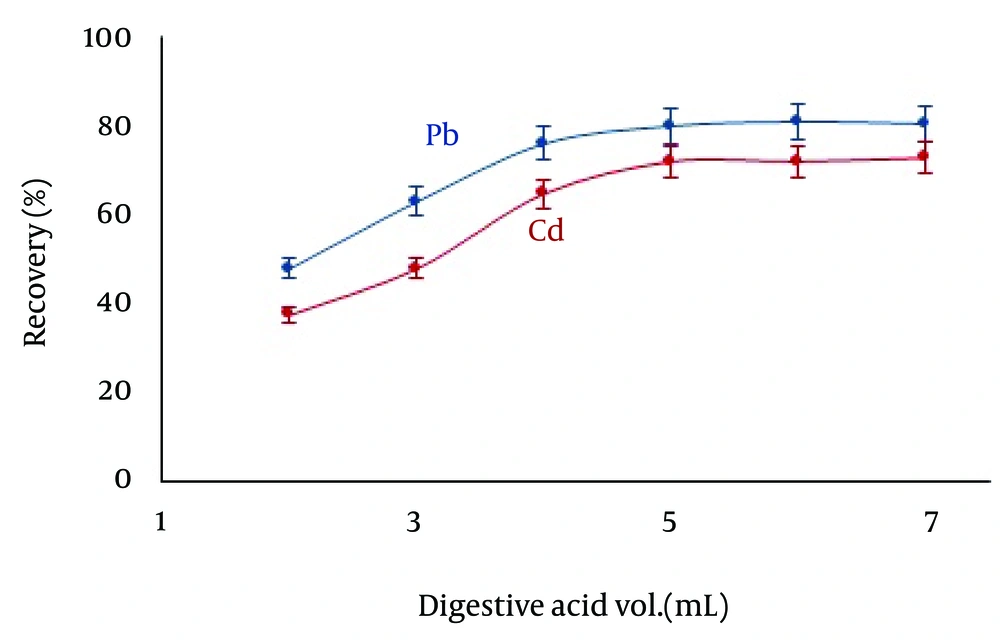
4.10. Effect of Volume of Digesting Solution
To attain the optimum volume to digest, after the adsorption process on the 100-mL sample solution (pH = 7.0, 25°C, Pb(II) 0.1 mg/L, Cd 0.05 mg/L, 40 mg ZVINPs, contact time: two minutes, 300 rpm), the ZVINPs were settled by magnet. Preconcentration and determination processes of Pb and Cd ions were studied by different volumes of a mixture of nitric acid:sulfuric acid (4:1) as the best digesting solution, ranging 2-7 mL. The best recoveries of Pb2+ (80%) and Cd2+ (72%) were attained when 5 mL of the digestive solution was used (Figure 8).
4.11. Real Samples
The investigated magnetic solid phase extraction method was used for the removal of Pb and Cd ions in water sample solutions. To specify the usability of the method, several 50 mL Pb(II) and Cd(II)-contaminated solutions (spiked 1 and 5 mg/L of each) were prepared using different water samples with different matrices and the removal process was carried out at optimum conditions. The removal percentage of the analytes was evaluated using GFAAS and FAAS techniques. As shown in Table 2, the capability of the investigated method was at an acceptable level for Pb ions removal in several samples at different concentrations, ranging 92.1 - 99.9%. Additionally, the removal percentage of Cd was ranging 53.4 - 99.2%. According to the experiments on river and Well water samples, the efficiency of Cd(II) removal process by ZVINPs was approximately as well as Pb ions removal. However, the removal efficiency of Cd(II) decreased for the sea samples (53.4 - 64.1%). The removal of Cd at complicated matrices encounters with problems due to the high electrolyte effect on the removal process for cadmium.
| Removal, % | ||||
|---|---|---|---|---|
| Added Pb, mg/L | Added Cd, mg/L | |||
| Sample | 1.0 | 5.0 | 1.0 | 5.0 |
| Caspian Sea | 99.3 ± 0.3 | 99.1 ± 0.2 | 64.1 ± 0.9 | 61.5 ± 1.1 |
| Persian Gulf | 96.2 ± 0.2 | 92.1 ± 0.4 | 58.1 ± 1.3 | 53.4 ± 1.5 |
| Karoon River | 99.8 ± 0.1 | 99.5 ± 0.2 | 96.1 ± 0.6 | 94.3 ± 0.8 |
| Well water | 99.9 ± 0.1 | 99.8 ± 0.2 | 99.2 ± 0.4 | 98.9 ± 0.7 |
4.12. Analytical Performance
Under the optimum conditions described, the flame atomic absorption spectrophotometric calibration curves were linear over the concentration ranges of 0.051 - 1.219 mg/L and 0.006 - 0.136 mg/L for Pb and Cd ions, respectively. The limit of detection (LOD) of the proposed method for determination of the desired Pb and Cd was studied under the optimal experimental conditions. For 99% confidence limit, LOD was obtained from LOD = 3.3 SDblank/m, where SDblank is the standard deviation of the signal of blank at 10 times measurements and m is the slope of the calibration curve at optimized conditions. The LODs were 0.015 and 0.002 mg/L for Pb2+ and Cd2+, respectively. The repeatability of the method was assessed by analyzing the same samples 10 times. RSDs of 3.7% and 4.6% were achieved for mean Pb and Cd ions concentrations of 0.3 and 0.03 mg/L, respectively.
4.13. Analytical Application
To investigate the applicability of the proposed procedure to real samples, it was applied for preconcentration and determination of Pb and Cd ions from 100 mL of spiked Karoon River samples. Table 3 shows the capability of the method for determination of Pb and Cd ions in different concentrations in aqueous solutions. The results of the three analyses of each sample showed that the adsorption percentages of Pb and Cd ions were almost quantitative. The enrichment factor, defined as the ratio between the analyte concentrations determined in the acidic digested solution and the initial concentration of analytes in the sample, were more than 16 and 14 for Pb2+ and Cd2+ for the analyzed spiked samples, respectively.
| Pb(II) | Cd(II) | ||||
|---|---|---|---|---|---|
| Added (mg/L) | Recovery (%) | EF | Added (mg/L) | Recovery (%) | EF |
| 0.10 | 80 | 16.00 | 0.01 | 70 | 14.00 |
| 0.50 | 83 | 16.60 | 0.10 | 72 | 14.50 |
| 1.00 | 81 | 16.24 | 0.50 | 72 | 14.36 |
5. Discussion
A simple magnetic solid phase extraction of Pb(II) and Cd(II) from aqueous solutions was successfully investigated with ZVINPs as adsorbents. Adsorption behavior could be described by Langmuir isotherm. This adsorbent is also useful for the removal of other heavy metal ions and organic pollutants from aqueous solutions. The whole removal (adsorption) process can be completed within two minutes. In addition, the proposed procedure is applicable in a wide pH range from 2.0 to 8.5. Table 4 shows a comparison between some parameters of previously reported methods as well as the proposed method. The proposed method showed high potential for removal of Pb(II) and Cd(II) from water samples. Table 5 shows a comparison between this method as a method for preconcentration and determination of Pb and Cd and some previously represented methods. As shown, this method has acceptable specifications for trace determination of Pb and Cd ions in water samples.
Moreover, the experiments showed that this magnetic SPE method can be used for preconcentration and determination of Pb and Cd ions in water samples. This SPE method has acceptable LODs for the analyzed ions in this study and is precise for determination of Pb and Cd (RSD% < 5). In addition, the enrichment factors evaluated for Pb and Cd ions were more than 16 and 14, respectively.
| Adsorbent | qm, mg/g | Time, min | Removal, % | Reference | |
|---|---|---|---|---|---|
| Pb | Cd | ||||
| Pine cone | 27.5 | 29.7 | 105 | 89 | (17) |
| Poly(aryl ether ketone)-PEK-L | 67.1 | 69.9 | 10 | ~95 | (18) |
| Titanate nanotubes | 520.8 | 238.6 | 180 | ~100 | (19) |
| Kaolinite clay | 0.7 | 0.6 | 30 | 85 | (20) |
| Vanadium mine tailing | 8.8 | 2.8 | 5 | - | (21) |
| Turkish illitic clay | 238.9 | 11.2 | 240 | ~100 | (22) |
| Manganese dioxide | 917.0 | 176.0 | 60 | ~100 | (23) |
| Neem oil cake | 30.0 | 15.0 | - | 98 | (24) |
| Polymer-supported nanosized Fe(III) oxides | 332.0 | 157.0 | 130 | ~100 | (25) |
| ZVINPs | 96.5 | 58.3 | 2 | ~100 | this |
Comparison of Some Parameters of Studied Removal Procedure With Some Reported Methods in Literature a
Comparison of Some Parameters of the Studied Method for Preconcentration and Determination of Pb and Cd with Some Reported Methods in the Literature a