1. Background
Styrene (ST) is a widely used organic solvent. This chemical is used in the production of many products including polymers which are incorporated into products such as plastic, rubber, fiberglass and food containers. Styrene exposure has been associated with numerous health effects in human and experimental animals. Occupational exposure to this agent can cause fatigue, memory lost, and liver plus kidney damage (1, 2). Moreover, ST may be absorbed into blood stream by all routes of administrations (2-6). Hepatotoxicity and nephrotoxicity of ST are the most common concerns among workers exposed to this chemical. The liver plays a major role in the biotransformation of the xenobiotic. Numerous studies have identified ST- induced hepatotoxicity in humans (2, 7-9). Kidney damage was also noted in workers occupationally exposed to ST (10, 11). The metabolites of ST observed in the urine of rats and humans (12-14). This has led to demand for to study laboratory animals to be used in the early diagnosis and prevention of occupational diseases.
A large body of evidence indicated that ST is metabolized by cytochrome p450 to reactive toxic intermediate mainly Styrene Oxide (SO). Covalent binding of SO to essential macromolecules produces cell injury (15, 16). Styrene metabolite decreased plasma GSH levels in mice (17). ST induced cell injury (18). These authors found that Glutathione (GSH), N-Acetylcysteine (NAC) protected liver against ST-induced toxicity in experimental animals (18). Vitamin C (Vit C) plays an important role as an antioxidant to protect cells against free radical produced injury. This chemical has ability to scavenge free radicals and also protecting cell membrane by regenerating the antioxidant (19, 20). Investigation regarding the effect of Vit C on ST-induced toxicity may be useful for better understanding of the clinical pictures following ST exposures in humans.
2. Objectives
For a better understanding of ST toxicity, this study was undertaken to determine the preventive effect of Vit C on styrene-induced toxicity in rat liver and kidney.
3. Materials and Methods
Adult male wistar rats (250-300 g) were housed in groups of 3 in clear polypropylene cages in a light cycle (12 h light and 12 h dark) and temperature-controlled room. The animals were allowed food and tap water ad libitum. The animals were pretreated with an intraperitoneal injection of 300 mg/kg Vit C (21). Control rats received vehicle only (distilled water, D H2O). After 30 minutes, animals were given different doses (0, 200, 400, or 600 mg/kg, ip) of ST (15). After 24 hours, all animals were killed with over dose of sodium pentobarbital. The animals' blood sample was collected for determination of biochemical parameters. Liver damage was estimated by measuring serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) activity. Nephrotoxicity was evaluated by measuring blood urea nitrogen (BUN) and creatinine (CR) levels. The liver and kidney tissues were removed, fixed and processed for light microscopy. The tissue samples were fixed in 10% buffered formalin for 24 hours, routinely processed and paraffin embedded. Five histological sections each at least 15 µm apart were taken from each tissue block and stained with hematoxylin and eosin (H and E). The protocol was approved by the ethics committee of the Ahvaz Jundishapur University of Medical Sciences.
Biochemical data were expressed as mean ± standard error. The data were analyzed using analysis of variance (ANOVA), a completely randomized design, and treatment differences were identified using the Newman-Keuls method. P value < 0.05 was considered as the criterion for significance. Five animals were used for each treatment group.
4. Results
A dose-related increase in biochemical parameters was observed in ST-treated when compared to control animals. Vitamin C had no effect on blood biochemical parameters. However, the AST, ALT, and ALP levels were significantly (P < 0.05) decreased in rats received various doses of ST and Vit C when compared to those which received ST only at the same dose (Table 1).
| Parameters | Treatment | Dose, mg/kg | |||
|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | ||
| AST | |||||
| ST | 30.9 ± 1.67 | 71.1 ± 3.32 c | 77.8 ± 2.94 c | 86.6 ± 2.56 c | |
| VC + ST | 31.1 ± 1.67 | 59.4 ± 4.73 | 67.8 ± 1.85 | 78.2 ± 3.31 | |
| ALT | |||||
| ST | 29.4 ± 4.97 | 37.4 ± 4.82 | 39.2 ± 3.72 c | 47.6 ± 3.89 c | |
| VC + ST | 28.3 ± 4.93 | 36.4 ± 4.39 | 30.8 ± 2.38 | 38.4 ± 2.89 | |
| ALP | |||||
| ST | 97.3 ± 4.54 | 107.8 ± 2.14 c | 122.2 ± 3.75 c | 147.4 ± 3.67 c | |
| VC + ST | 96.2 ± 4.2 | 99.8 ± 2.28 | 102.2 ± 3.75 | 124.3 ± 4.79 | |
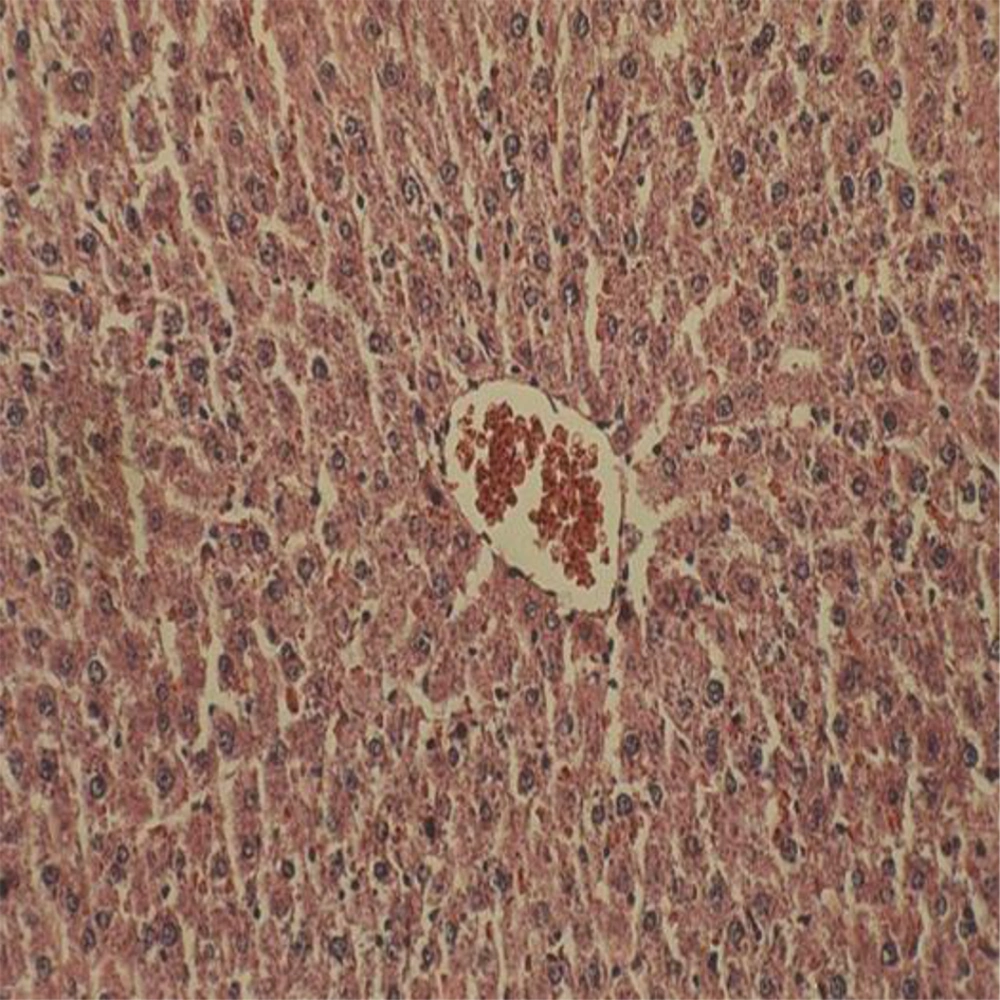
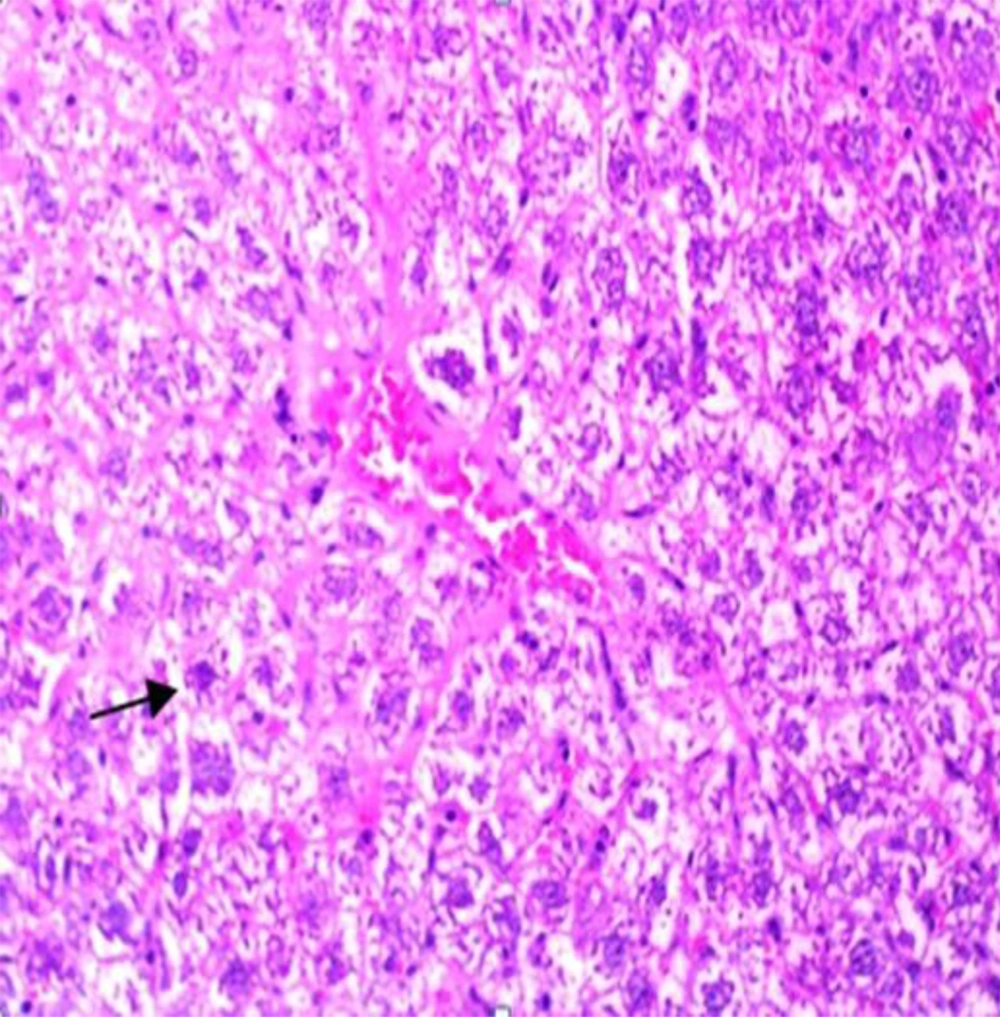
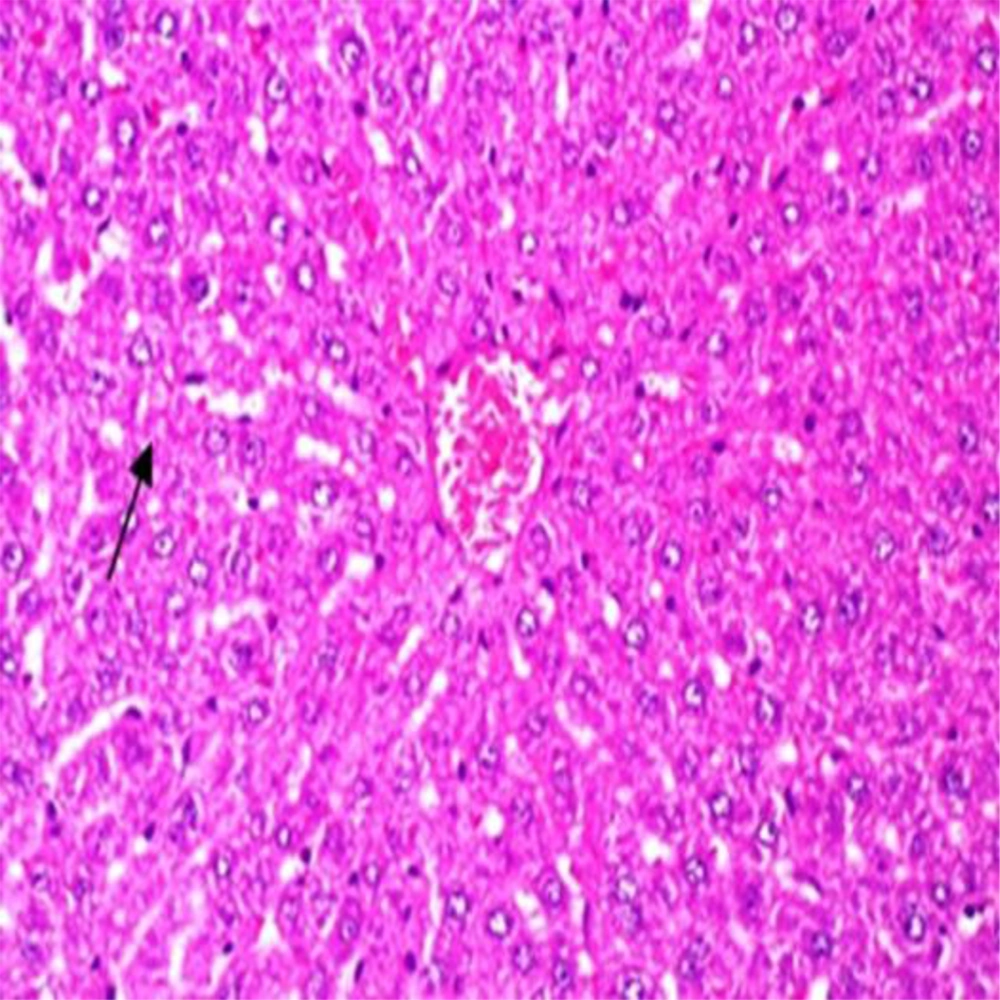
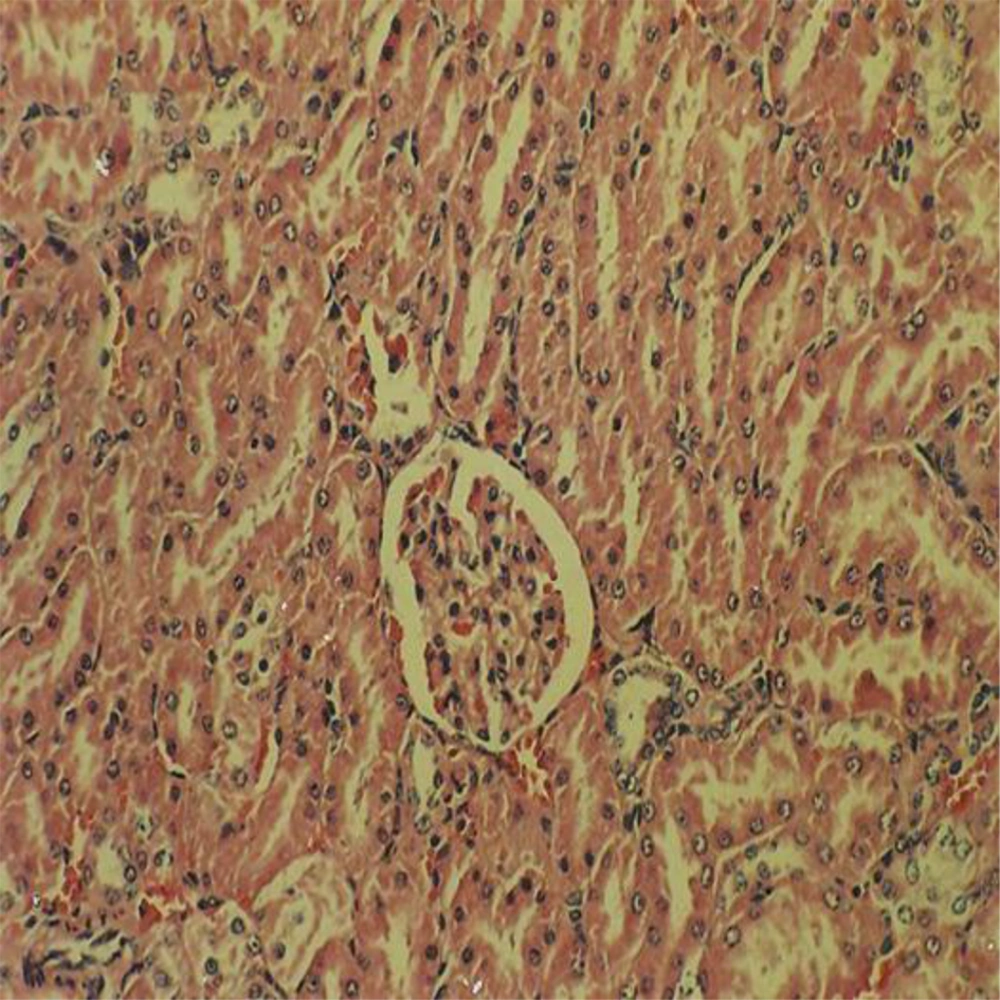
Administration of vehicle alone did not produce detectable injury in rat liver (Figure 1). In control rats (vehicle-treated animals) liver cells were intact. There was no detectable injury in hepatocytes (Figure 1). However, ST induced injury in the liver. The extent of injury appeared to be a dose- and time-related manner. However, the most remarkable histopathological alterations were noted in rats treated with 600 mg/kg ST. The liver cells were intensively swollen. The vessels of portal space and centrilobular vein being distended and congested with hemolyzed material. The hepatocytes were enlarged and granular with many vacuoles. The nuclei were appeared to be larger. Hydropic degeneration was obvious (Figure 2). Vitamin C had no effect on liver tissue and there was no obvious injury. However, this agent protected hepatocytes against ST-produced cytotoxicity (Figure 3).
Showing the ST-induced liver injury was markedly diminished in comparison with non-pretreated animals which received the same dose of this chemical (Figure 2).
A dose-related increase in BUN and CR concentrations was observed in ST-treated animals compared to those in the control group. Elevation of renal biochemical parameters was predominant in rats treated with 600 mg/kg ST (Table 2).
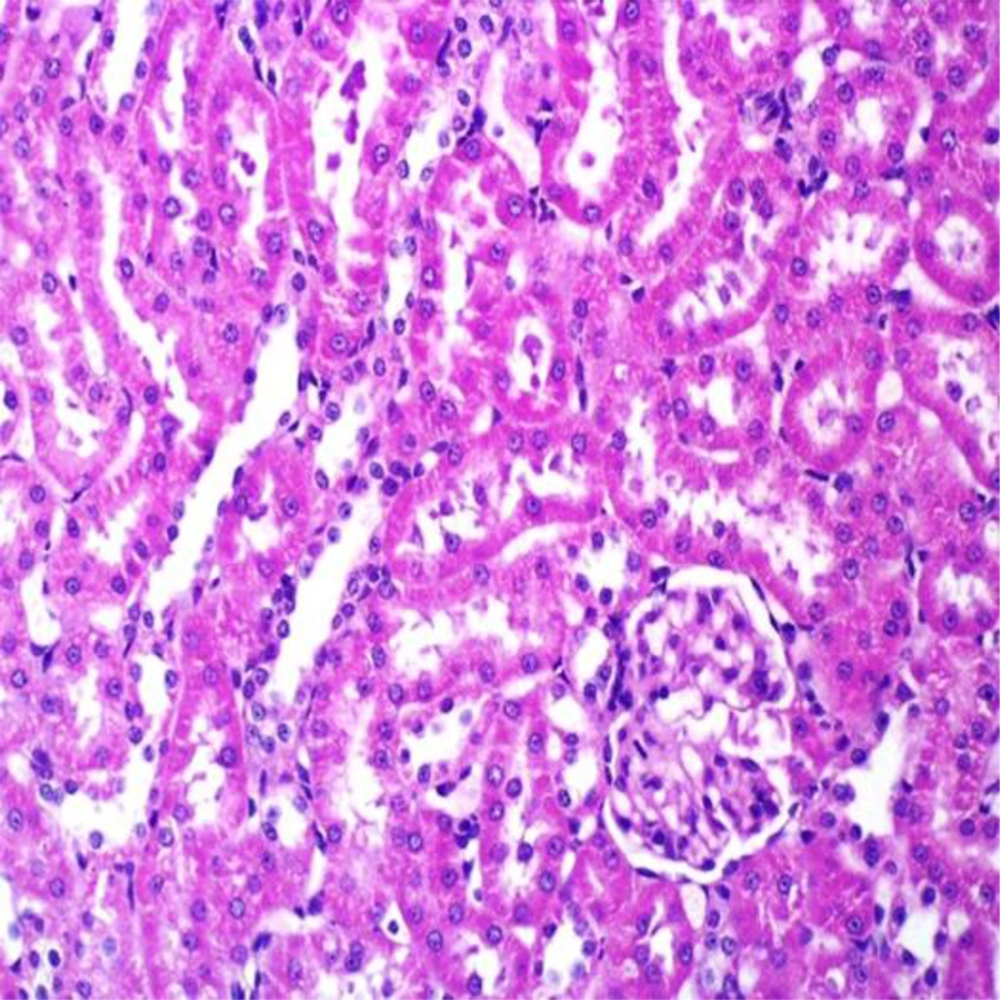
Vitamin C had no effect on BUN and CR when compared to control animals. However, pretreated animals with this agent markedly reduced the level of biochemical parameters when compared to ST-treated rats (Table 2). Administration of vehicle alone did not produce detectable injury in rat kidney (Figure 4). However, a dose-related injury in the ST-treated rats was noted. Light microscopy revealed that renal tubular cells were swollen, had loss of staining capacity, and nuclei appeared to be dilated, presence of blood clot was noted in the ST-treated rats (Figure 5). Similarly, Vit C had no effect on kidney cells. However, the extent of ST-induced injury was decreased in Vit C pretreated rats when compared to those treated with ST only (Figure 6).
Showing ST-induced kidney injury was markedly diminished in comparison with non-pretreated animals which received the same dose of this chemical (Figure 5).
5. Discussion
The liver is the main organ for xenobiotic biotransformation. Human hepatotoxicity of ST concern over occupational hazards associated with this chemical led to considerable interest to study of this agent on experimental animals. We found that ST induced dose-dependent elevations of ALT, AST and alkaline phophatase in rat liver. The effect of ST on experimental animals was reported by several investigators (7, 15, 22, 23). Hirasawa et al. (2007) reported that serum AST and ALT activities were significantly increased in rats treated with ST (22). Elevation of ALT and AST was noted in workers exposed to ST (24, 25).
Dose-related histopathological alterations were noted in rat liver treated with ST. we observed damage mainly in centrilobular area in rat liver. Similarly, short-term inhalation of mice to ST caused necrosis of centrilobular hepatocytes (26).
The mechanism by which ST produced toxicity is not completely understood. The initial step in the pathway leading to ST toxicity is generally assumed to be the biotransformation of ST to a reactive intermediate, possibly Styrene Oxide (SO) by cytochrome p450 (15). The presence of cytochrome p450 in the liver as well as kidney in various animal species has been described by many investigators (15, 27, 28).
The kidneys play a major role in the elimination of xenobiotics from the bloodstream. Nephrotoxicity is one of the most common adverse effects of toxic chemicals. A large body of evidence indicated that ST caused nephrotoxicity (10, 11, 29). An increased albumin excretion was noted in workers exposed to ST (10, 11). We observed that BUN and CR increased in a dose-dependent manner in ST-treated rats when compared with vehicle-treated animals. These results suggest an impairment of renal functional parameters due to ST exposure. Tubular injury in kidney of rat treated with ST was reported as evidenced by increasing of beta 2-microglobulinuria (30). Significant urinary excretion of gamma-glutamyltranspeptidase and proteins were noted in ST-treated rats (31). Our findings along with others suggested that kidney is susceptible for ST-induced toxicity. We observed that ST produced damage mainly in proximal convoluted tubular cells.
Since kidneys usually have low drug-metabolizing enzyme activities, chemically induced nephrotoxicity has been assumed to be produced by toxic intermediate (s) generated in the liver and transported to the kidney. As ST is eliminated via the kidney, another possibility for ST caused renal damage is that translocation of ST metabolites from the liver to the kidney via general circulation produced kidney injury. However, the generation of metabolites in kidney may at least in part be responsible for kidney toxicity.
Vitamin C is an antioxidant in cells, which has relatively high rate constant of its reaction with free radicals. It has been found that ST reduced level of Vit C and enhanced ST toxicity. Administration of Vit C protected rat against ST-induced lung injury (31, 32).
Our results indicated that ST induced injury in rat liver and kidney. Pretreatment of animals with Vit C markedly decreased ST hepatotoxicity and nephrotoxicity. These findings suggested that Vit C protected liver and kidney cells against ST toxicity and support the view that these organs have ability to metabolize ST and induced oxidative stress. On the basis of these results, we conclude that Vit C may prevent the occurrence of ST-induced adverse effects in humans.