1. Background
Due to the rapid increase in the population and the shortage of water resources in recent years, human beings have seriously threatened the wildlife by polluting the water resources. Municipal and industrial wastewaters are among the pollutants of the environment, especially water resources. By entering the surface waters, these pollutants not only endanger human’s health and the environment, but also cause biological problems for animals and plants (1). Nowadays, there are a wide variety of wastewater treatment systems; however, sequencing batch reactor (SBR) seems to be the most promising and appropriate modified activated sludge process which can be utilized to remove carbon and nitrogen organic matters (2-5). In recent years, SBR has been widely used to treat industrial and municipal wastewater because of its low cost and suitable efficiency in pollutant removal (6-8). The process is composed of five stages as filling, reaction, settling, effluent and idle (9, 10). The intermittent cycle extension aeration system (ICEAS) or advanced SBR process is a variant of an SBR system where the processes of biological oxidation, nitrification, phosphorous removal and liquids/solids separation can be achieved continuously in a single tank. What makes the ICEAS process different is a continuous inflow, even during the settle and decant phases of the operating cycle. Wastewater flow continuously fills up the reactor and discontinuously discharges (11). In this process, wastewater enters the reactor and after physical and biological processes exits. To improve the performance of the process, two or more reactors with predetermined orders are utilized. All the necessary processes in this method are chronologically conducted in a pool. Through alternate use of aerobic and anaerobic processes in the SBR system, large amounts of organic matter and nutrients can be removed (12, 13). The SBRs performance is satisfactory in treating domestic wastewater. The quality of effluent has been reported 20 and 5 mg/L for chemical oxygen demand (COD) and biochemical oxygen demand 5 (BOD5), respectively, by Lamine et al. (14). Zhou et al. (15) studied the capability of SBR in treating landfill leachate. The study resulted in up to 94% and 98% of COD and BOD5 removal. In another study by Goncalves et al. (16) a SBR unit operated for organic removal from wool dyeing effluent. COD and BOD5 removal rates were 85% and 95%, respectively. The SBR system can be used in biological treatment of different types of industrial and municipal wastewaters (17). Different studies have been conducted on treatment of biological wastewater of olive oil industry (18), wastewaters containing antibiotics (19), removal of cyanide from wastewater of electroplating industry (20), textile industry wastewater (21), wastewaters containing phenol (22), coal industry wastewaters (23), wastewaters with high concentrations of nitrogen (12), and domestic wastewaters and waste leachate (24).
More than 100,000 synthetic chemicals such as detergents are used in a variety of domestic and industrial applications and numerous studies have documented that many of these compounds are incompletely degraded or removed during wastewater treatment and are persistent in the aquatic environment (25). Detergents are a group of chemicals that have cleansing properties. These compounds have a polar hydrophilic group and a nonpolar hydrocarbon branch (hydrophobic) (26). These compounds enter surface waters through domestic and industrial wastewaters and cause environmental hazards. Linear alkyl benzene sulfonates (LAS) are the most important ionic detergents which produce negative ions in the environment. LASs are utilized in household detergents like washing powder, dishwashing detergent, etc.(17, 18). In aquatic environment, detergents float on the surface of water as a surface layer and disfigure the aquatic environment, reduce gas exchange and endanger the aquatic animals’ health by decreasing the dissolved oxygen. These compounds change the taste and smell of the water, produce fume on the surface of water, cause disruption in the process of water and wastewater treatment, increase the treatment costs, and lead to aquatic animals’ death (27-30).
2. Objectives
Due to the appropriate properties of the SBR system for wastewater treatment and the inefficiency of other wastewater treatment systems of Yazd in removing detergents, Yazd’s company of water and wastewater has designed an Advanced SBR system to treat the municipal wastewater. In this regard, the present study was conducted to determine the performance of Advanced SBR in Yazd wastewater treatment plant to remove organic materials and detergents.
3. Materials and Methods
3.1. Wastewater Characteristics
Raw wastewater with the following qualitative and quantitative properties was fed to the SBR system in the Yazd wastewater treatment plant.
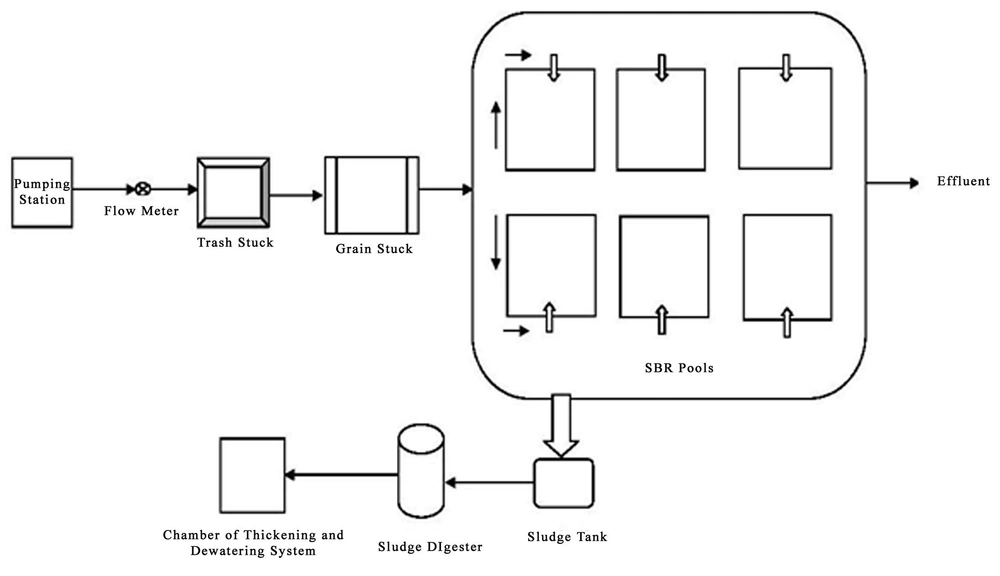
3.2. The Schematic of Yazd Wastewater Treatment Plant
In this system, before wastewater enters the SBR system, it passes two initial treatment units consisting of a screening and a grit chamber and is then discharged into the reactors. In this process, six SBRs are simultaneously employed. The dimension of each reactor was as follows: the length 40 meters, the width 23.75, and the depth 6.6 meters. Afterwards, inside each reactor, mixing operation with a retention time of 0.9 hour and aeration operation with a retention time of 2.5 hours get conducted. When the biological treatment ends, sedimentation operation with a retention time of one hour begins and lasts until complete discharge of clarified effluent with a retention time of half an hour. All of these phases are conducted in one reactor and the total retention time is 4.9 hours. Extra sludge is also discharged from the system in each treatment period.
3.3. Sampling and Experiments
The present research was a descriptive longitudinal study conducted over eight months. Composite sampling was carried out on a daily basis from October 2012 to June 2013. In this regard, special containers (1 L) for wastewater sampling were utilized to collect samples from the input and output of the SBR system in Yazd wastewater treatment plant. Therefore, six samples per month and 48 samples during the eight-month period from the output and 48 samples from the input of the plant, equal to a total of 96 samples were collected for further analysis and examination.
The experiments conducted on the samples included BOD5, COD, and detergents (LAS). After sampling, the samples were taken to the chemistry laboratory of the faculty of health, Shahid Sadoughi university of medical sciences, Yazd, and the abovementioned parameters were measured according to the guidelines of the standard methods (31). Due to the importance of measuring the detergents, the measurement method is explained below.
The utilized materials and instruments included chloroform, sodium hydroxide, phenolphthalein index solution, standard LAS solution, 1 N sulfuric acid, standard stock solution of LAS, detergent, and separating funnels. First, the sample wastewaters were spilled into the separating funnels, and then by adding drops of 1 N NaOH and using 10 drops of phenolphthalein, the solutions became alkaline. The pink color was removed by adding drops of H2SO4. To prevent the formation of emulsion, 10 mL chloroform, 25 mL blue methylene and 10 mL isopropyl alcohol were added to the samples. The funnels were shaken for 30 minutes so that the phases would separate. By tilting the funnels and opening their valves slowly, the accumulated pressure was released. Some samples needed more time to separate. Before the chloroform layers were discharged, the samples were shaken slowly so that they could settle. Therefore, the chloroform phase placed at the bottom and the aqueous phase positioned on top. After the layers separated, the chloroform layer was spilled into another separating funnel and the first funnel tube was washed with some chloroform. Afterwards, by adding 10 mL of chloroform in each cycle, the extraction was repeated three times. All of the resulting substances from the chloroform extraction were spilled in the second funnel; then, 50 mL detergent solution was added and the funnel was severely shaken for 30 minutes. No emulsion was formed in this solution. After sedimentation, the contents were shaken and the chloroform layer, separated from the aqueous layer, was transmitted to a 10-mL volumetric flask. The detergent solution was again extracted using 10 mL chloroform and the result was added to the previous volumetric flask. This process was repeated once more to make sure about the precision of the experiment and the volume of the extracted chloroform reached 100 mL after it was spilled into the volumetric flask. Afterwards, the cap of the flask was replaced and it was slightly turned to prevent sedimentation. Prior to the final measurements, the flask was turned several times to mix the contents. The absorptions of the standard samples were measured against the control chloroform at 652 nm using a spectrophotometer. Using the prepared standard absorption rate and concentration, the calibration curve was drawn and the LAS concentration was measured (31). Finally, the collected data were analyzed through analysis of variance (ANOVA) using software package for statistical analysis (SPSS) version 18.0.
4. Results
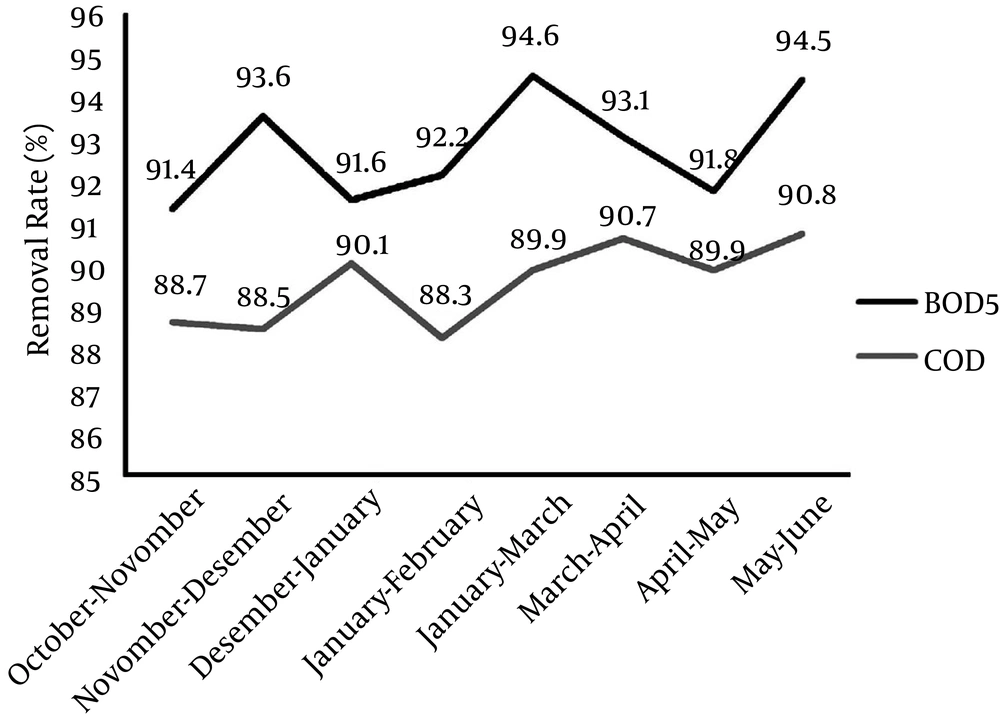
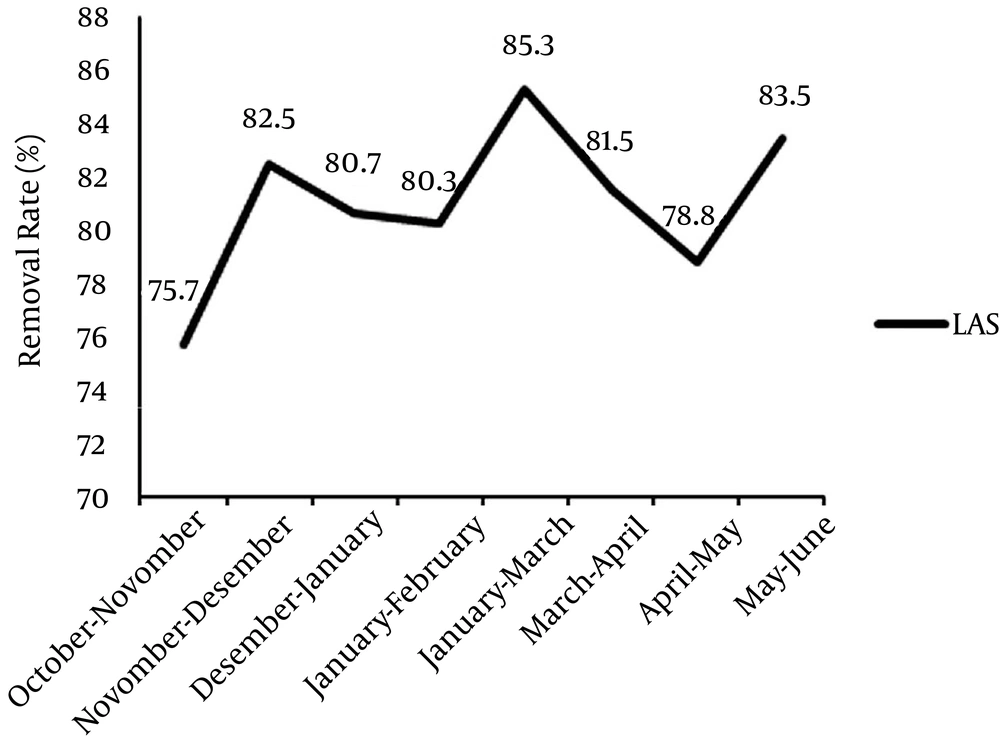
The results of the experiments of input and output BOD5, COD and detergents are presented in Tables 2 and 3. The removal percentages of these parameters are indicated in Figures 1 and 2.
The results indicated that during the eight-month period, the removal efficiencies of BOD5 and COD were 92.95% and 90.06%, respectively. ANOVA also showed that there was a significant relationship between the input and output means of BOD5 and COD (P ≤ 0.001).
| Parameter | Mean |
|---|---|
| BOD, mg/L | 232 |
| Population | 150,000 |
| Mean flow rate, m3/d | 31950 |
| Maximum flow rate per hour, m3/h | 2743 |
| TSS, mg/L | 277 |
| TKN, mg/L | 40 |
| TP, mg/L | 8 |
Quantitative Properties of the Raw Wastewater Entering Yazd Wastewater Treatment Plant a
| Months | BOD5, mg/L | COD, mg/L | ||
|---|---|---|---|---|
| Input | Output | Input | Output | |
| October - November | 268.21 ± 40.10 | 23.10 ± 3.94 | 524.10 ± 91.34 | 59.2 ± 9.53 |
| November - December | 237.77 ± 33.91 | 15.02 ± 2.85 | 549.31 ± 93.82 | 41.3 ± 7.82 |
| December - January | 251.12 ± 37.22 | 21.12 ± 3.64 | 563.12 ± 95.15 | 62.7 ± 9.61 |
| January - February | 261.50 ± 38.33 | 20.2 ± 3.40 | 511.70 ± 90.78 | 56.2 ± 8.73 |
| February - March | 324.29 ± 50.75 | 17.40 ± 2.92 | 639.50 ± 99.77 | 59.4 ± 9.41 |
| March - April | 393.76 ± 63.17 | 25.31 ± 4.41 | 814.04 ± 112.23 | 64.3 ± 10.75 |
| April - May | 259.35 ± 43.21 | 24.20 ± 4.12 | 563.42 ± 96.12 | 75.1 ± 11.31 |
| May - June | 357.23 ± 55.61 | 18.40 ± 3.10 | 612.08 ± 99.22 | 56.4 ± 8.66 |
| Total Mean | 292.40 ± 45.28 | 20.59 ± 3.54 | 597.15 ± 97.30 | 59.34 ± 9.47 |
| Months | Detergent (LAS), mg/L | |
|---|---|---|
| Input | Output | |
| October - November | 2.5 ± 0.79 | 0.61 ± 0.096 |
| November - December | 2.4 ± 0.82 | 0.42 ± 0.075 |
| December - January | 3.1 ± 0.93 | 0.6 ± 0.094 |
| January - February | 3.16 ± 0.90 | 0.62 ± 0.082 |
| February - March | 5.3 ± 1.11 | 0.78 ± 0.16 |
| March - April | 3.2 ± 0.96 | 0.59 ± 0.088 |
| April - May | 2.9 ± 0.84 | 0.62 ± 0.091 |
| May - June | 3.7 ± 1.01 | 0.61 ± 0.089 |
| Total mean | 3.29 ± 0.92 | 0.60 ± 0.096 |
The results indicated that the total mean of the detergents decreased from 3.29 to 0.6. ANOVA also showed that there was a significant relationship between the input and output means of the detergents (P ≤ 0.001). Moreover, the mean removal rate of the detergents was 81.6% (Table 3).
In this study, the mean input of BOD5 was 292.40 ± 45.28 and its output was 20.59 ± 3.54 mg/L. The mean input and output of COD were respectively 597.15 ± 97.30 and 59.34 ± 9.47 mg/L. Moreover, based on LAS, the mean input and output of the detergents were 3.29 ± 0.92 and 0.606 ± 0.09 mg/L, respectively (Table 4). Finally, the removal efficiencies of BOD5, COD and detergents were respectively 92.95, 90.06 and 81.6%.
| Parameters | Values b | Output Standards | ||
|---|---|---|---|---|
| Discharge Into Surface Waters | Discharge Into Absorbent Wells | No Restriction to Irrigation Use | ||
| BOD5 | 20.59 ± 3.54 | 30 | 30 | 100 |
| COD | 59.34 ± 9.47 | 60 | 60 | 200 |
| Detergent (LAS) | 0.606 ± 0.09 | 1.5 | 1.5 | 1.5 |
5. Discussion
The results of the study by Fernandes et al. (2) on domestic wastewater treatment using SBR process with limited air indicated that the removal efficiency of COD was 83% which is lower than the corresponding value of the present study. This difference can be attributed to different air strategies employed in the two studies (2).
Ghahfarrokhi et al. (34) conducted a study on removal of detergents from hospital wastewaters using SBR method and indicated removal efficiencies of 94.54%, 92.97% and 84.99% for BOD5, COD and detergents, respectively. These results are in agreement with those of the present study and indicate that in appropriate conditions, SBR system can remove more than 90% of BOD5 and COD and more than 80% of detergents from wastewaters (34). In a study on removal efficiency of BOD5, COD and detergents, Ehrampoush et al. (35) concluded that artificial subsurface constructed wetland system has lower removal efficiency compared to the SBR system.
Other studies investigated the use of the SBR method in treating different types of industrial wastewaters; however, their results indicated lower removal efficiency of COD compared to the present study (4, 20, 23, 24). Therefore, the SBR system performs better in treating municipal wastewaters that contain organic matters with higher biodegradability compared to industrial wastewaters.
In a study by Pirsaheb et al. (36) the average of LAS removal in extended aeration process in winter and summer were 94.06% and 99.23%, respectively. In a study by Duarte et al. degradation of LAS in anaerobic SBR (ASBR) was evaluated. Degradation of the surfactant at the end of the operation was 87% and removal of chemical oxygen demand was 86% (37). One study encompassed a total of 50 municipal wastewater sites and included 15 activated sludge systems, 12 trickling filters, six oxidation ditches, eight lagoons, and nine rotating biological contactors (RBC). The influent concentrations of LAS for all the treatment plants showed a normal distribution with a mean of 5 mg/L. The average effluent LAS concentrations ranged from 0.04 mg/L for activated sludge plants to about 1 mg/L for trickling filter plants. A range of removal rates over 99% for activated sludge treatment (11). LAS removal from wastewater by aerobic processes in well-designed municipal wastewater treatment plants was above 90% (38). As observed, detergents removal efficiency in our system was a little less than other systems that may have been caused by the operation of the wastewater treatment plant in undesirable condition (36).
The presence of surfactants in the influent could cause floc saponification, which decreases the floc dimensions and increases the floc circularity. This results in poor sludge settling and washout of biomass in the system (38). In a study by Rittmann et al. (39) they found that when LAS stays in solution it will rapidly degrade, but slow desorption of LAS initially sorbed to the sludge occurs, which may limit biodegradation rates due to limited bioavailability. Therefore, by increasing the retention time in the aeration stage, the removal efficiency of LAS will increase.
The collected data indicated that the removal efficiencies of BOD5, COD and detergents were lower in cold months compared to warm ones and this is consistent with Pirsaheb et al. results (36). This can be attributed to the fact that microorganisms are less active in cold environments; therefore, removal efficiency in wastewater treatment systems drops and rises as temperature falls and increases (40).
With the proper operation of the wastewater treatment plant and increasing the retention time, the removal efficiency of detergents increases. According to the environmental standards of BOD5, COD and detergents issued by the Department of Environment (32), the results of the present study indicated that the outputs of these parameters were appropriate and standard for agricultural and irrigation uses.