1. Background
In recent years, research on advanced contaminant treatment processes in aquatic environments has become more common, in particular, advanced oxidation processes are important because of their efficiency (1). Studies show that advanced oxidation processes that are highly reactive in aquatic environment (based on the formation of free radicals) have high ability in the destruction of high strength wastes under optimal operation (2, 3). Performance of advanced oxidation processes are highly dependent on the oxidation conditions such as reaction time, pH range of the environment, type and concentration of oxidizing and oxidized compounds, and use of chemical and physical factors generating hydroxyl radicals in the process (4, 5). The results of the studies in this area indicate that under identical conditions, the oxidation resistance of different chemical compounds differs from each other and depends on the nature and structure of chemical pollutants (2). Kinetics of chemical oxidation reactions of pollutants can be important in achieving optimal conditions and determining the oxidation reaction of chemicals with respect to time and different operating conditions (6).
In the past decade, zero-valent iron (ZVI) is considered as an effective agent for treating organic compounds in aquatic environments (7). However, studies have shown that ZVI reactivity significantly increases by reducing the size at the nanoscale (nZVI). The reactivity of iron nanoparticles is 500 to 1000 times higher than iron powder (8), because iron nanoparticles (nZVI) become corroded in acidic conditions, and generate iron ions. These ions in the presence of hydrogen peroxide cause oxidation of contaminants through Fenton process (3).
Removal mechanism of contaminants using ZVI is the direct transfer of electrons from ZVI to contaminant compounds that result in conversion of pollutants to non-toxic or less toxic compounds. On the other hand, ZVI can oxidize and decompose contaminants in the presence of soluble oxygen as the result of transferring of two electrons to O2 molecule and producing H2O2. Hydrogen peroxide formed during this process can transfer two other electrons from ZVI to produce water molecule. In addition, H2O2 and Fe2+ synthesis (fenton process) can lead to the formation of hydroxyl radicals (OH0) as the most important factor of organic compounds oxidation (Equations 1 - 3)



High standard reduction potential of ZVI makes the restoration work to be carried out in an appropriate manner. During this process, iron oxide (Equation 4) and alkyl aldehydes are restoring (Equation 5). The process take place in a very favorable thermodynamic condition (Equation 6) (9).

Equation 5.RX +H+→ RH +X-

In addition, ZVI produces hydrogen gas and hydroxide ion as a result of reaction with water, which leads to an increase in pH during water oxidation process (Equation 7) (10).

Ultrasonic waves accelerate chemical or biological processes in water and wastewater treatment operations. When the pressure increases, the tensile strength of the fluid in low pressure or diluted areas creates tiny vapor bubbles, known as cavitation, which creates a strong mechanical and sonochemical effects, which convert suspension particles and large molecules in the liquid, into smaller particles (11, 12).
Combining ultrasound and Fenton (sono-Fenton) is one of the most widely used advanced oxidation processes in the treatment of organic pollutants from wastewater. In Fenton process, the produced Fe2+ ions react with hydrogen peroxide and produce hydroxyl radicals (reaction 2). In this reaction, OH0 attacks organic molecules and destroys their structure during the process of radical oxidation, hydrogen abstraction, electron transfer, and radical integration. These processes are presented in chemical equations 8 to 11, respectively (13, 14).




Analysis of MTBE kinetics is highly dependent on the concentration of hydroxyl radicals produced in the oxidation process, the latter of which depends on the type of chemical oxidation process and the nature of the oxidizing agent. Contact time, MTBE initial concentration, and temperature are other factors involved in MTBE decomposition kinetics (15). The oxidation process system is also effective on removal kinetics. According to the study by Boonrattanakij et al. on the kinetics of 6, 2-dimethyl aniline in batch and continuous systems, the batch system shows better performance compared to the continuous system in similar working conditions (16). In the study by Hwang et al. on MTBE oxidation via Fenton-like method, at initial concentration of 11 mM MTBE, 6 mM H2O2, and 5 mM Fe2+, after 90 minutes only 45% of the initial MTBE concentration was removed. These differences in the effect of oxidation time can be attributed to the use of nZVI and its role in accelerating the oxidation process (17).
2. Objectives
Given the environmental importance of MTBE and the impact of various factors on the performance of MTBE oxidation process using advanced oxidation (including environmental pH as the most important factor influencing the process based on free radical formation), the primary objective of this study was to evaluate the effect of pH changes on the removal kinetics of this compound using H2O2/nZVI/ultrasonic process and its impact on the reaction rates.
3. Materials and Methods
3.1. Chemical Compounds
Methyl tertiary butyl ether ((CH3)3 COCH3) compound with the purity of 99.9% and hydrogen peroxide (H2O2) with a purity of 30%, n-Pentane, toluene, hydrochloric acid, and sodium hydroxide with laboratory purity were purchased from Merk Company.
3.2. Nanoparticles Synthesis
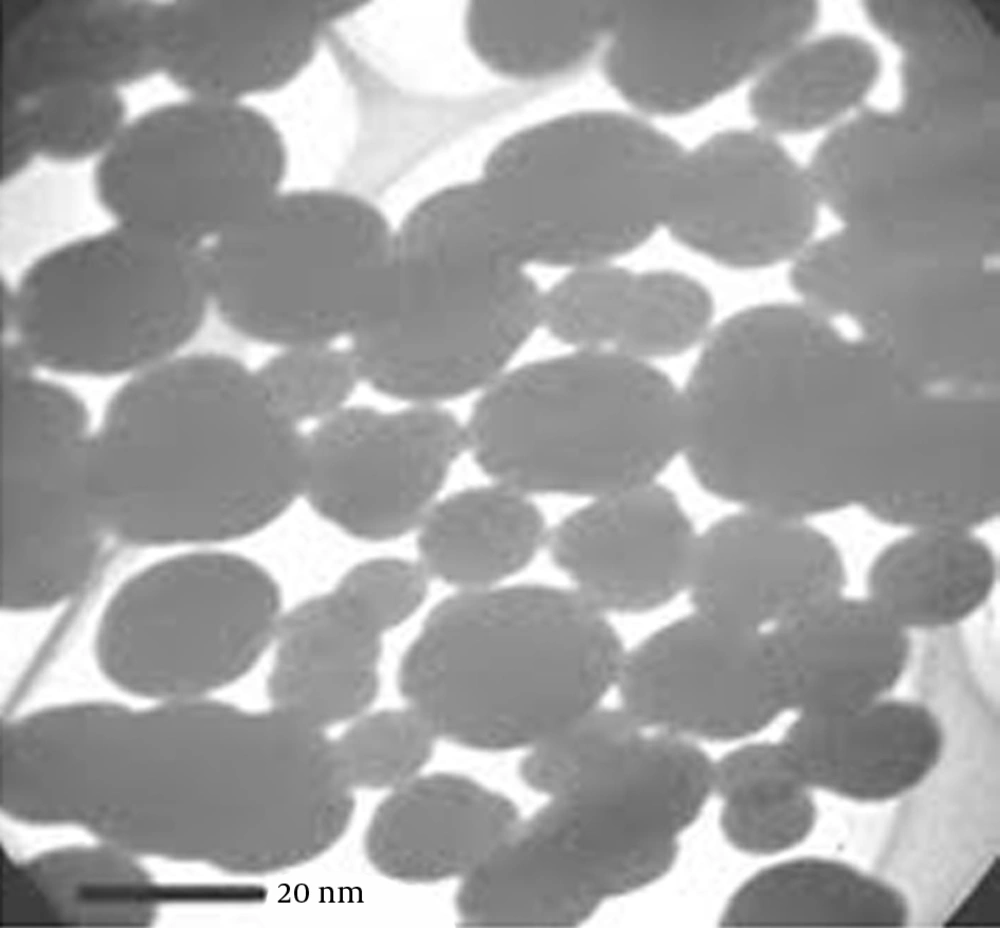
Iron nanoparticles used in this study were synthesized using sodium borohydride. During the process of synthesis, nanoparticles were synthesized by adding sodium borohydride solution (0.1 M) to ferric chloride solution (FeCl3 .6H2O) at room temperature. Ferric chloride solution was prepared by dissolving 0.5406 g of solid FeCl3.6H2O in ethanol water solution with a volume ratio of 4/1 (4 parts ethanol to one part water). To prepare a solution of sodium borohydride, sodium hydroxide 0.1 was used. After preparing the solutions, sodium borohydride solution was added and dropped (one drop every 2 seconds) in severe mixing conditions (in vacuum) to ferric chloride solution. With the addition of the first few drops of sodium borohydride solution, black colored solid particles (iron particles) quickly appeared. This condition lasted about 30 minutes. The vacuum filtration method was used for the separation of the liquid phase from black iron nanoparticles. The isolated nanoparticles were washed 3 times with 25 mL ethanol to remove all water. This can be considered as the most important phase of NZVI synthesis, because prevents rapid oxidation of NZVI. Eventually, the washed nanoparticles were put in the oven overnight and dried at 50°C. In order to prevent oxidation in air, nanoparticles were stored in ethanol solution (8, 18). To determine the properties of produced iron nanoparticles, TEM electron microscope was used (Figure 1).
3.3. Methodology
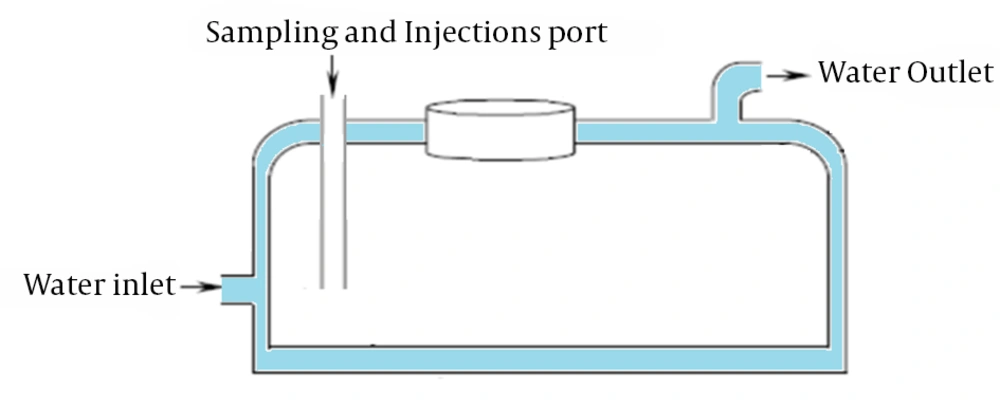
The reactor used in the process was Plax glass double layered with 1 L capacity (Figure 2), which was placed in the Elmasonic-S60h model ultrasonic device with a constant frequency of 60 Hz and 500 kW power. First, 1 L of co-distilled water was poured into the reactor. Then, based on the variables and research objectives, one factor was considered as variable, and others kept as constants. To adjust the temperature (30°C), cooling water in the reactor chamber was used. After sampling, the samples were placed in a centrifuge device at 4000 rpm for 5 minutes. All samples were collected in glass containers with Teflon cap and kept at 4°C. To adjust the pH, 1 N hydrochloric acid, and 1 N sodium hydroxide were used. N-Pentane was used as an extraction agent and toluene as an internal standard. In MTBE removal via H2O2/nZVI/ultrasonic process, the effects of some parameters such as contact time (2 to 60 minutes), concentration of hydrogen peroxide (5 to 20 mL/L), concentrations of nZVI (0.15 to 0.45 g/L), MTBE concentrations (50 to 750 mg/L), and pH (2 to 9) were investigated. The samples were measured using GC-FID device to evaluate the residual amount of MTBE. Chromatographic conditions were as follows: detector temperature: 250°C, temperature at injection point: 180°C, column temperature: 32°C, column temperature increase gradient: 6°C/min, column high temperature: 115°C, and initial time: 2 minutes. Standard curves for MTBE concentration was drawn, and the remaining concentration was calculated using the curve.
4. Results
In this study, MTBE oxidation was studied using H2O2/nZVI/Ultrasonic process at different pH levels. For this study, concentrations of MTBE, H2O2, and NZVI were fixed at 500 mg/L, 10 mL/L, and 0.25 g/L, respectively. Each experiment was repeated twice and the data shown in the charts are based on average data for each stage of the test. The pH values in this study were 2, 3.5, 5, 7, and 9.
4.1. Effect of pH Changes on MTBE Removal Efficiency
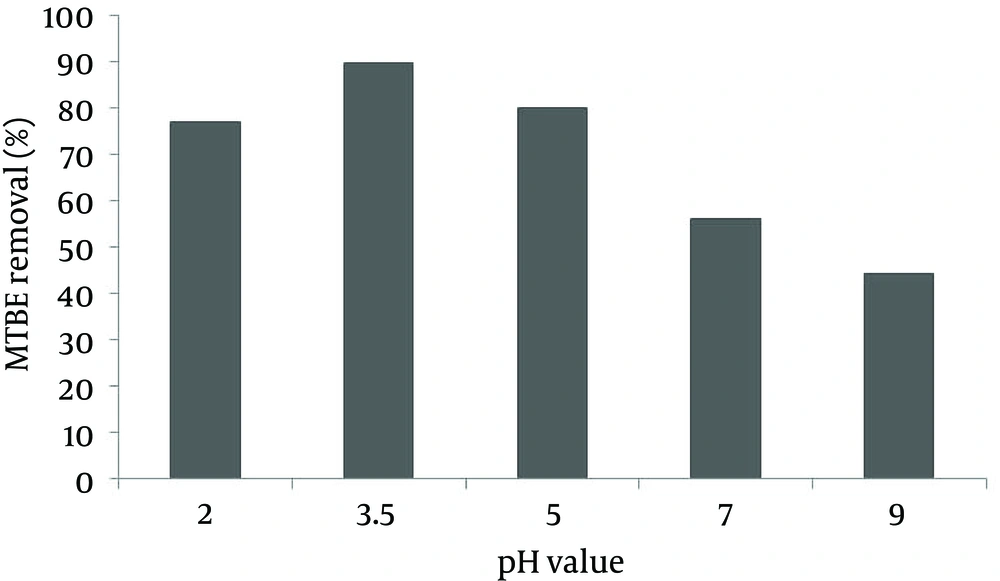
In oxidation conditions of 10 mL/L H2O2, 0.25 g/L nZVI , and 50 mg/L MTBE, removal efficiency at pH = 3.5 was about 89.56%, and in the same conditions, at pH = 9 it reduced to 44.14%. According to the results, the oxidation under acidic conditions was better compared to neutral and alkaline conditions. However, the acidity level of the oxidation environment was also important. So that the performance at pH = 2 was lower than pH = 3.5, and was equal to 76.93% (Figure 3).
4.2. Effect of pH Changes on the Rate of MTBE Elimination in Unit of Time
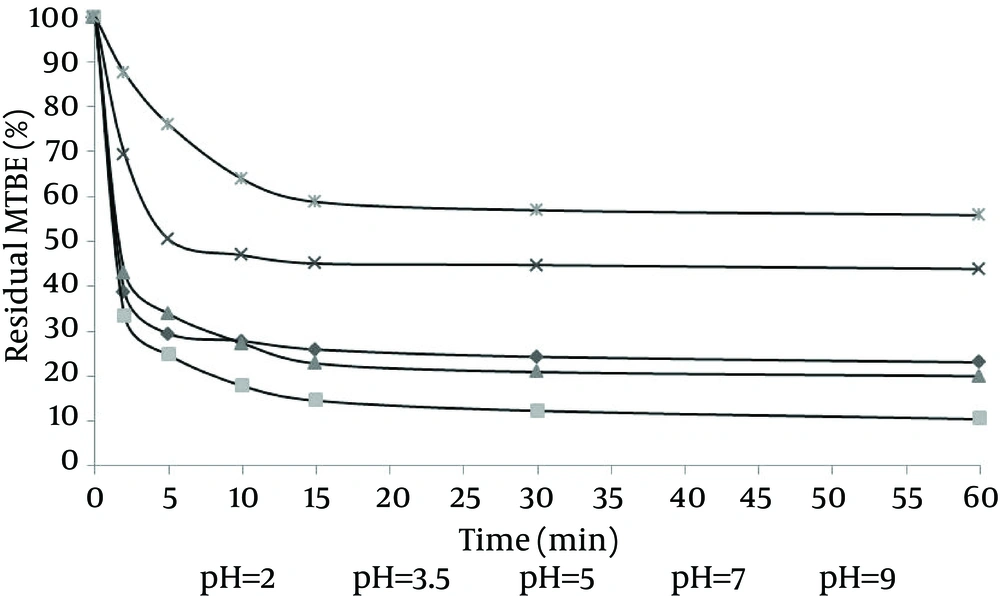
Study of the effects of time on H2O2/nZVI/ultrasonic oxidation method showed that MTBE concentration undergoes significant changes quickly and in the early minutes of oxidation. According to the results, oxidation rate was very high in the first minutes after the start of the process (especially the first 10 minutes). The results of oxidation in H2O2 concentration = 10 mL/L, nZVI = 0.25 g/L, MTBE = 500 mg/L, and in pH values of 2, 3.5, 5, 7, and 9 are shown in Figure 4. The results showed that the percentage of remaining MTBE, 60 minutes after the beginning of oxidation and in pH values of 2, 3.5, 5, 7, and 9 were 23.07%, 10.44%, 19.99%, 43.88, and 56.86%, respectively.
4.3. MTBE Oxidation Rate Values in the Test Optimal Conditions
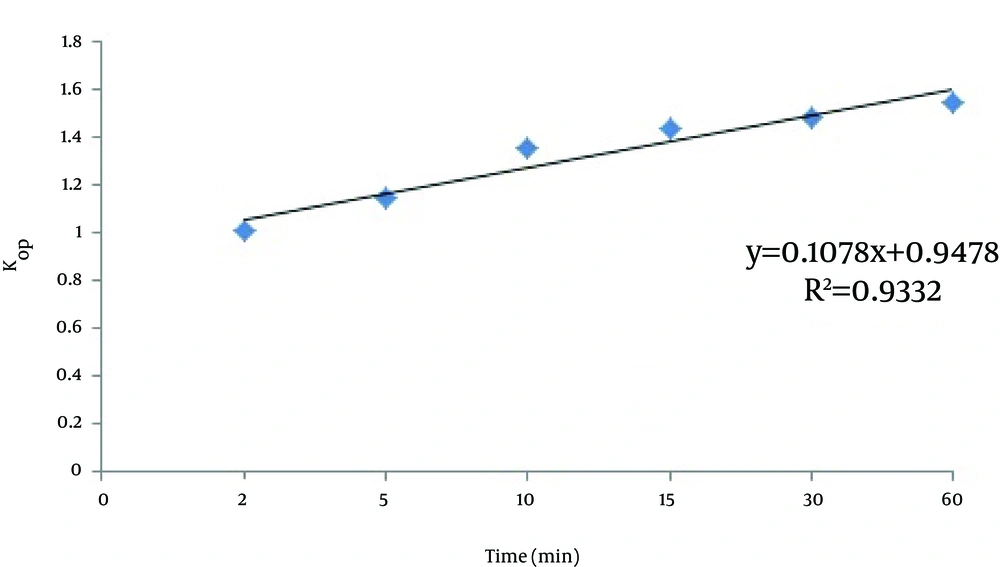
To determine the effect of pH changes on MTBE decomposition constant rate (kap), oxidation tests with the initial concentration of H2O2, nZVI, and MTBE as 10 mL/L, 0.25 g/L, and 500 mg/L were performed. In addition, the levels of k (min-1) were calculated through the following kinetic equation and the results are shown in Table 1.


Where:
C0: initial MTBE concentration at time t = 0 (mg/L)
Ct: MTBE Concentration at time t
k: constant kinetic rate of first-order reactions (min-1 )
t: reaction time (min)
According to Table 1 and Figure 5, the MTBE decomposition constant rate (kap) increases over time, and at any time it depends on the amount of oxidation and the optimum condition of the oxidation environment. According to Table 1, the highest value obtained for kap was achieved at pH 3.5.
| Effect of pH Changes on Initial Logarithmic Mtbe Concentration Changes at The Unit of Time | Time, min | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 10 | 15 | 30 | 60 | |
| Ln (MTBE/MTBE), pH = 2 | 0 | 0.951141 | 1.222476276 | 1.277978218 | 1.348998576 | 1.414282398 | 1.466637114 |
| Kap | 0 | 0.475571 | 0.244495 | 0.127798 | 0.089933 | 0.047143 | 0.024444 |
| Ln (MTBE/MTBE), pH = 3.5 | 0 | 1.098412 | 1.398771883 | 1.724848764 | 1.928266714 | 2.097198263 | 2.259525604 |
| Kap | 0 | 1.004836 | 1.144212 | 1.34967 | 1.429406 | 1.482871 | 1.540617 |
| Ln (MTBE/MTBE), pH = 5 | 0 | 0.841879 | 1.082640519 | 1.297185186 | 1.477532842 | 1.564464547 | 1.609938037 |
| Kap | 0 | 0.766451 | 0.773994 | 0.752057 | 0.766249 | 0.745978 | 0.712512 |
| Ln (MTBE/MTBE), pH = 7 | 0 | 0.365716 | 0.684187439 | 0.75608698 | 0.796509694 | 0.80430223 | 0.823711551 |
| Kap | 0 | 0.434404 | 0.631962 | 0.582867 | 0.539081 | 0.514107 | 0.511642 |
| Ln (MTBE/MTBE), pH = 9 | 0 | 0.133303 | 0.274568433 | 0.448476999 | 0.530688253 | 0.562820919 | 0.582321625 |
| Kap | 0 | 0.364499 | 0.401306 | 0.593155 | 0.666267 | 0.699763 | 0.706948 |
Logarithmic Equation of Oxidation Reaction and Decomposition Rate of MTBE per Unit of Time (Kap)
5. Discussion
The effect of pH changes on oxidation kinetics and removal of MTBE composition using H2O2/nZVI/ultrasonic oxidation process was studied. In previous studies in the field of fenton process, it was shown that low pH is highly effective on oxidation process performance (19, 20).
The results of the current study show that in acidic pH condition, removal efficiency is higher than neutral or alkaline conditions. During the oxidation process, pH undergoes changes through the time, and because of the production of H+ ion in test environment (Equation 14), it decreases gradually. These pH changes causes the MTBE removal changes curve to be different in various pH ranges (21).
Our results on the effect of pH are consistent with the studies of Burbano et al. In their study, which was on the effect of pH on MTBE removal in Fenton process, different values of pH from acidic to neutral was studied, and eventually it became clear that the highest MTBE removal efficiency occurred in the pH range of 3 to 3.5 (6). Darban et al. studied the reduction of MTBE concentration in water using Fenton method and determined the changes resulting from reaction conditions in producing byproducts. The results showed that MTBE removal efficiency was about 99.99% at pH = 3 (22). Bergendahl et al. carried out Fenton oxidation process using ZVI, and found that 99% of MTBE at acidic pH was degraded at H2O2: MTBE optimum ratio of 220: 1 (23).
The high efficiency of oxidation under acidic conditions might be due to the high level of surface oxidation of nanoparticles in acidic conditions; Fe2+ ion concentration increases rapidly in the environment and provides the conditions for greater amounts of MTBE oxidation in a short period of time. In these situations, generating ultrasonic waves in the fluid greatly helps higher concentration of Fe2+. Also, due to Fenton chemistry, by increasing pH, various types of iron are deposited as sediment in the form of Fe (OH)3, causing H2O2 to break down into O2. On the other hand, by reducing pH under strongly acidic condition, Fe (III) turns into Fe (II). Both of these conditions lead to reduction in concentration of the oxidizing agents (24).

According to the results of this study, the highest removed concentration of this compound is in the first 15 minutes. After this time, the removal efficiency shows little change. The results indicate that due to high speed and capacity of hydroxyl radicals in MTBE oxidation, the optimal required time for oxidation of the compound is relatively low.
Kinetic study of the MTBE concentration at unit of time, show that MTBE oxidation somewhat follows the first-order oxidation reactions. Kinetic constant of MTBE concentration changes per unit of time (kap) and shows partial increase during oxidation process. The results also show that under optimum conditions in terms of pH, the value of kap is higher than other pH values, and lower level of kap in high pH is obtained, which is due to remarkable decline in hydroxyl radicals as well as insoluble iron oxide deposits in alkaline pH.
In this study, the role of pH (acidic, neutral, and alkaline) on MTBE oxidation kinetics using H2O2/nZVI/ultrasonic advanced oxidation method was investigated. The results showed that pH could remarkably affect the efficiency and oxidation rate of MTBE. Under identical conditions, the amount of kap in terms of oxidation declines substantially by moving away from the optimum pH range. In this study, pH = 3.5 is considered as the optimal pH in H2O2/nZVI/ultrasonic oxidation process, with the elimination of about 89.56% of the high MTBE concentration. Generally, by adjusting pH in this range, the rate and efficiency of MTBE oxidation can be enhanced in H2O2/nZVI/Ultrasonic method.