1. Background
Recently, nitrate concentration in water resources has increased due to a significant decrease in precipitation, excessive use of fertilizer and untreated domestic wastewater (1-3). Based on a study in 2004 – 2007, out of the 27 monitoring stations in the European Union, two thirds had average nitrate concentrations below 25 mg/L and only 15% had average nitrate concentrations above 50 mg/L in ground water. In surface water, 21%, 37%, 3%, and 3% of the stations had average nitrate concentrations below 2, 2 - 10, 40 - 50, and 50 mg/L, respectively (4). One study showed that two thirds of groundwater resources had average nitrate concentrations of 50 mg/L during four consecutive years (2004 – 2007) in Europe (4).
There are many health concerns for drinking water with high levels of nitrates concentration, especially for children younger than 6 (2).
Physico-chemical treatments are commonly used to improve the quality of drinking water (5). In general, many treatment methods are used for the removal of nitrate from water including ion exchange, reverse osmosis, and biological denitrification (6). Reverse osmosis and ion exchange cannot selectively remove nitrate and the presence of interfering ions decreased the nitrate removal efficiency (7). Electrocoagulation is the process of destabilizing suspended, emulsified, or dissolved contaminants in an aqueous medium by introducing an electric current (6). In the process of electrocoagulation, a coagulant is produced in the electrolytic cell using an appropriate anode and cathode (8). The advantages of this process are the simplicity of the operation, higher sludge settling ability and relatively lower cost. Electrocoagulation process has been applied efficiently for the remediation of groundwater and surface water (9, 10). Recently, the electrocoagulation technology is used for the treatment of municipal and hospital wastewater (11, 12). Many organic pollutants are mostly converted into harmless byproducts using the electrochemical processes (13). In the electrocoagulation process, the electricity is passed directly from the sacrificial electrodes (mostly iron and aluminum) inside a reactor tank where the current produces a coagulating agent (14). This process has three stages: 1) coagulants formation due to anode electrical oxidation, 2) destabilizing pollutants and suspended substances and emulsion breaking and 3) combining instable particles to form flock (15, 16).
The main reactions occur at iron electrodes are as follows (17):
Anode: Fe (s) → Fe+2 (aq) + 2e-
Fe+2 (aq) + 2OH- (aq) → Fe (OH)2 (s)
Cathode: 2H2O (l) + 2e- → H2 (g) + 2OH- (aq)
Overall: Fe (s) + 2H2O (l) → Fe (OH)2 (s) + H2 (g)
Also, when aluminum is used as an electrode the reactions are as follows (18, 19):
Anode: Al (s) → Al+3 (aq) + 3e-
Cathode: 3H2O (l) + 3e- → 3/2H2 (g) + 3OH- (aq)
Overall: Al+3 (aq) + 3H2O (l) → Al (OH)3 (s) + 3H+ (aq)
Kumar et al. showed that the removal efficiency of nitrate and arsenic from drinking water was increased by increasing reaction time and the applied voltage using EC process (8). Raghu Prasad et al. found that by using an electrolytic reduction method for nitrate removal from ground water, the optimal conditions of operation were found at pH = 8, at 0.89 mA/cm2 for 7 h (20). Malakootian et al. showed that under optimum condition (pH = 7.34, electrical potential difference 40V, initial concentration of nitrate 100 mg/L, TDS=1.27g/L, iron electrode and 4 pairs of electrodes), removal of nitrate from Kerman water was 89.7 % (21).
2. Objectives
Since nitrate concentration in many groundwater resources in Iran has increased significantly, and an appropriate method is absolutely essential to meet the water quality standards. Therefore, the main objective of this study was to evaluate the feasibility of using the electrocoagulation process for nitrate removal in aqueous phase, and determine the optimal condition for the maximum removal efficiency.
3. Materials and Methods
All the experiments were performed at a bench-scale batch reactor at room temperature. Two-fold iron and aluminum electrodes were used in this study. A cube-shaped electrochemical Pyrex cell with the 10 mm thickness glass and 2.25 L volume with dimensions of 12 cm × 12 cm × 16 cm and the electrode iron and aluminum plate with dimensions of 12 cm × 10 cm × 2 mm was used. The distance between electrodes was 5 cm. The electrode was connected to a Direct Current (DC) power supply. Magnetic stirrer was used at a constant speed of 100 rpm for mixing. Control was also used for all the experiments.
All chemicals were purchased from Merck (Germany). The electrodes were cleaned using hydrochloric acid 15% before starting the tests. NaOH and H2SO4 were used for adjusting pH.
The effects of different parameters (pH, voltage, and reaction time) on the reduction rate of nitrate were determined at three replications. The studied parameters were operation time (30, 60, and 75 min), voltage (15, 20, and 30), and pH (3, 7, and 11). Therefore, a total of 81 samples were considered.
The residual nitrate was determined at wavelength of 220 nm using a spectrophotometer (DR- 5000) according to the standard method (22).
The optimal conditions of different parameters were determined according to nitrate removal efficiency, which was calculated according to the following formula:
Where:
E = nitrate removal efficiency
C0 = Influent nitrate concentration before the EC process
C = Effluent nitrate concentration after the EC process
3.1. Data Analysis
Data were analyzed using the SPSS software version 16, (Inc. Chicago, Illinois, USA) and Pearson’s correlation coefficient was used to analyze the relationship between these parameters.
4. Results
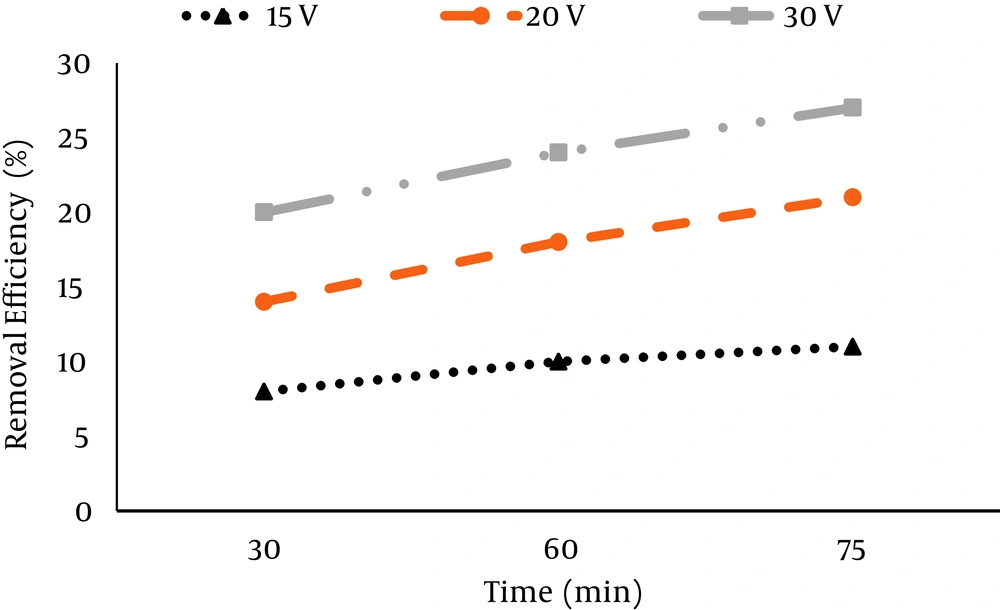
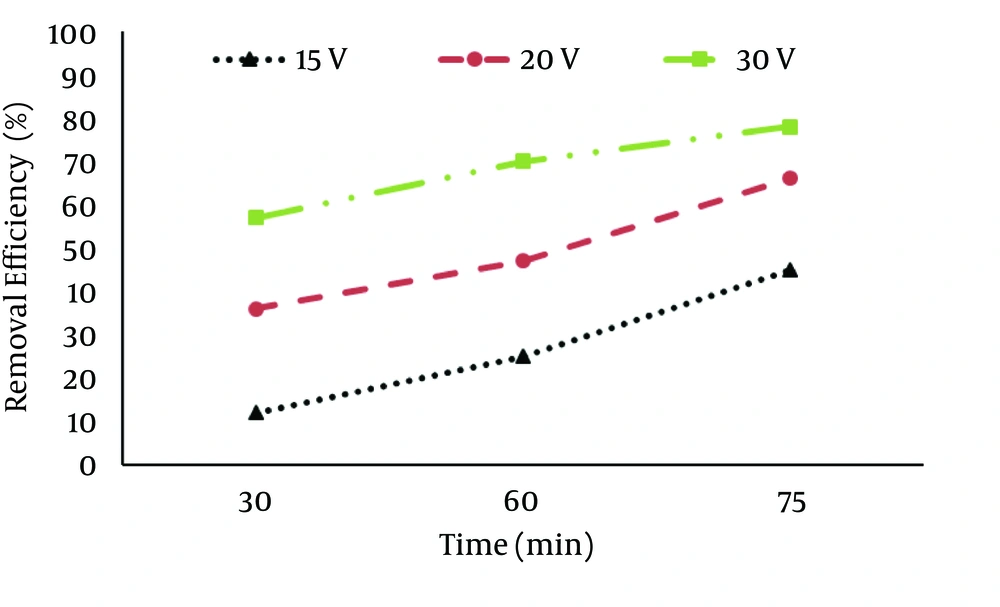
The results of nitrate removal by electrocoagulation process at different voltages of 15, 20, 30 V, operation times of 30, 60, and 75 min and pH (3, 7 and 11) are presented in Figures 1 - 3. According to Figure 1, the maximum removal efficiency of nitrate (27%) was at the voltage of 30 V, pH = 3 and operation time of 75 min, while the minimum removal efficiency of only 8% was at the voltage of 15 V at 30 min. According to Figure 2, at pH = 7, the maximum removal efficiency of nitrate occurred at voltage of 30 V and operation time of 75 minutes (78%), while the minimum removal efficiency occurred at voltage of 15 V at 30 minutes (12%).
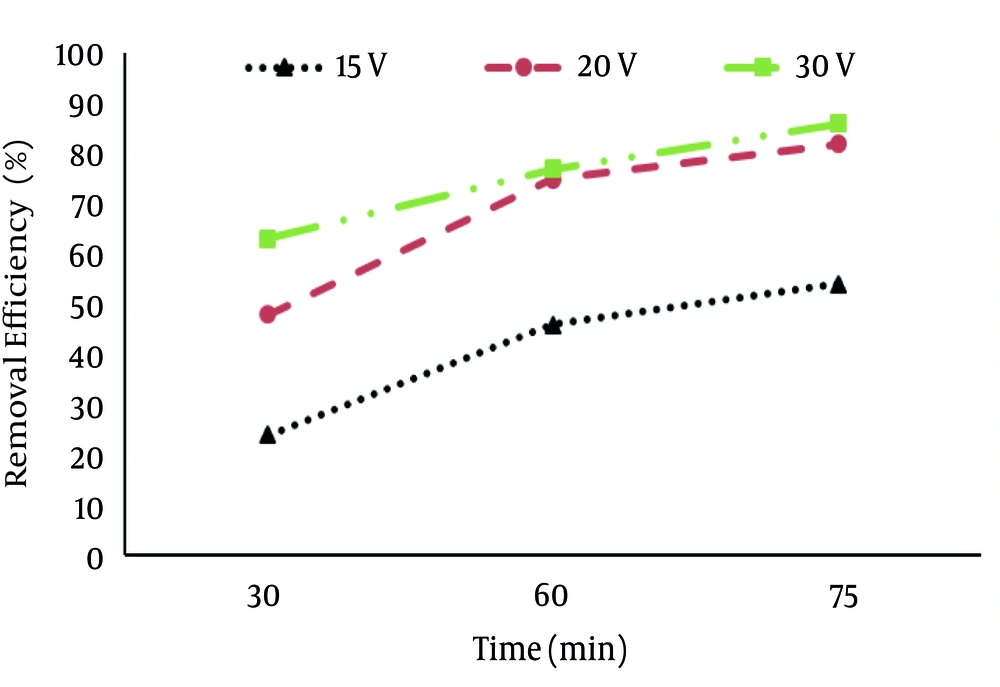
According to data obtained in the current study, the maximum removal efficiency (86%) of nitrate was at the optimal condition (30 V, pH of 11 and operation time of 75 min) and the minimum removal efficiency (24 %) was at voltage of 15 V at 30 min (Figure 3).
5. Discussion
The aim of this study was to assess the efficiency of nitrate removal using the electrocoagulation method in a batch system.
5.1. Effect of pH on Nitrate Removal Using the Electrocoagulation Process
pH has a significant effect on many chemical and biochemical reactions (23-25). Our results also demonstrated that any variation on pH had a tremendous effect on the removal of nitrate from the aqueous solution. By increasing pH from 3 to11, the efficiency of nitrate removal was increased by 59% using the EC method. The present study showed that the maximum nitrate removal (86%) was at pH = 11.
This can be due to a reaction between metal and hydroxide ions (21). Koparal on removal of nitrate from water by electroreduction and electrocoagulation has shown similar results (26). In contrast, Anbari et al. show a slight decrease in the removal efficiency of chromium when the initial pH is increased above 7 (27).
5.2. Effect of Reaction Time on Nitrate Removal Using the Electrocoagulation Process
Optimization of the operation time is a very important factor to reach the optimal condition for better removal of nitrate anion (28, 29). At acidic condition (pH = 3), increasing the reaction time had not a significant effect on the removal efficiency. However, in alkaline condition (pH = 11), the removal efficiency was increased significantly by increasing the operation time (P < 0.01).
Operation time has a major role in the electrocoagulation process. The removal efficiency was directly related to the concentration of ions generated on the electrodes. The ions' concentration was increased with increasing the time of electrolysis, which in turn caused hydroxide flocks to increase (11).
Many studies showed that by increasing the electrolysis time, the removal efficiency increases. Emamjomeh showed that pH levels of 9-11 were optimum for the removal of nitrate by EC (93%) (2).
5.3. Effect of Voltage on Nitrate Removal Using the Electrocoagulation Process
After determining the optimum conditions of pH and the operation time, the next step was to determine the effect of the operating voltage on the reduction rate of the nitrate. Our data showed that by increasing the applied voltage, the removal efficiency of nitrate was increased as well. Pearson’s correlation analysis showed that there was a significant relationship between the removal efficiency and voltage and also the same linear relationship was observed between the nitrate removal and the operation time (P < 0.01).
Many reports showed that by increasing the initial voltage, the corrosion rate of aluminum electrodes was also increased and produced more Al+3 ions and therefore increased the removal rate of nitrate. It can be attributed to more consumption of energy and then more production of flocks in shorter time (21). The results of different studies are consistent with our findings (30, 31). In another research by Mohammadi et al., nitrate removal was done using the EC methods. The results showed that -at electrical potential of 30 and 10 W, the nitrate removal rates were 88% and 38%, respectively (30). Similarly, Akhondi et al. conducted a study on the removal of cadmium by the EC method and demonstrated that the maximum removal efficiency of cadmium was at 185 W, operation time of 40 min, and pH of 10.25 (31). In another study the maximum removal efficiency of nitrate from drinking water was 84% at 25 V using electrocoagulation processes (8).
Hossini and Rezaee indicated that by applying the electric current of 0.14 A for 120 min, the nitrate content decreased to 97% (32). Furthermore, Adhoum et al. showed that the optimum current for the maximum removal of phenolic compounds from wastewater was at 75 mA/Cm2 (33).
Ghanim et al. (34) revealed that the removal of NO3- increased by increasing the electrolysis time using iron electrodes. At first, the removal of NO3- from aqueous solution was sharp, and then it increased very slowly until reach the equilibrium. More than 88% of NO3- was removed within 70 min by applying 10 mA/Cm2.
The present research aimed to assess the removal efficiency of nitrate using the EC method and the results are as follows:
The efficiency of this method in nitrate removal depends on voltage, contact time, and pH. The removal efficiency of nitrate more than 88% was observed at the optimal condition voltage of 30 V, operation time of 75 min, and pH of 11. Additionally, maximum removal efficiency was observed by increasing pH, time, and voltage. In conclusion, in comparison to the conventional treatment methods, the electrocoagulation process is a cost-effective method for removing nitrate from water resources to reach the water quality standard.