1. Background
World health organization (WHO) reported that in 2014, 9% of people 18 years and older had diabetes and in 2012, diabetes was the direct cause of 1.5 million deaths. In addition, more than 80% of diabetes death soccur in low- and middle-income countries and our country is classified in this category (1, 2). Insulin resistance is impaired glucose homeostasis in the presence of insulin andis related to many diseases such as hypertension, coronary artery disease, Alzheimer, and type 2 diabetes (3).
The endocrine system disruptions are the main factors in metabolic disorders such as insulin resistance and type 2 diabetes, which are due to lifestyle changes, obesity, and aging (4). Orexin or hypocretin is one of the hormones that affects the body metabolic processes. It was first discovered in the hypothalamus in 1998 simultaneously by two independent groups of researchers; one group named it orexin because of its regulating role in appetite and the other group named it hypocretin because it is secreted in hypothalamus (5, 6). This hormone is produced by a very small population of neurons located in and around the hypothalamic lateral nuclei (LH); however, the axons from these neurons extend throughout the brain and the spinal cord. Orexin is found in two types: orexin A (OXA) and orexin B (OXB), which are 33 and 28 amino acid residues long, respectively, and are made by the activity of the prepro-orexin (PPO) genes. Researches showed that OXA may be of greater biological importance than OXB (7).
OXA has an inevitable role in glucose metabolism, so that decrease in OXA leads to dysfunction, glucose tolerance and insulin resistance in non-obese male mice and almost obese female mice. OXA also improves the function of insulin receptors in the hypothalamus, and hyperglycemiatogether with increased peripheral insulin resistance reduce the expression of OXA, which in turn increases insulin sensitivity (8). Some studies have shown that physical activity can play an important role in the regulation of glucose homeostasis by affecting the adipoinsular axis and effective peptides on this axis such as OXA (9). A recent evidence indicated that the disruption of OXA production is an effective factor in glucose homeostasis and diabetes induced by ageing (10). Since OXA is a factor in integrating peripheral metabolism, central regulation of behaviors, and maintenance of energy homeostasis (11). It can be affected by energy homeostasis-changing factors. Given that physical activity increases the activity of cardiovascular, breathing and the energy-generating systems, it can affect the OXA secretion (12). This has been shown in several studies in order to evaluate the role of physical activity in OXA secretion of non-human subjects (9, 13). In addition, some studies reported no change in OXA levels after physical activity in animal specimens (13, 14).
There are a few studies that investigated the effect of physical activity on plasma levels of OXA in human samples. A recent study by Messina et al. (15) was conducted to investigate the effects of exercise on plasma levels of orexin in human samples. Subjects included six healthy non-athlete males with a mean age of 23 ± 3.12 years and mean body mass index (BMI) of 20 ± 1.9 kg/m2.The physical activity consisted of a cycle ergometer exercise at 75 W for 15 minutes. Blood sampling was done before, 15, and 30 minutes after the exercise. This was the first study that indicated physical activity and resulted in a significant increase in plasma levels of orexin in human specimens (15).
2. Objectives
There are a few studies that demonstrated the effects of physical activity on orexin changes, glucose homeostasis, and insulin resistance. Therefore, the present study was performed to evaluate the acute effect of aerobic exercise on plasma levels of OXA, insulin, glucose, and insulin resistance in males with type 2 diabetes.
3. Patients and Methods
Twenty patients with type 2 diabetes among the males admitted to Ahwaz Golestan hospital were selected using random sampling. They aged between 40 and 50 years and had conditions such as blood sugar in the range of 126 - 200 mg/dL, not smoking cigarette or any other drugs, no particular disease such as cardiovascular disease, respiratory or kidney disease, hypertension, as well as not using insulin and no diabetes complications such as peripheral vascular disease or diabetic foot ulcers. The subjects were randomly assigned into experimental and control groups, involving 10 people in each group. In brief, the participants were contacted and preparations such as completing a questionnaire on demographic information, explaining about the investigation, collecting their consents, explaining the cooperation schedule, understanding how to complete the dietary recalls questionnaire during the investigation etc. was done. Before and after the main activity, anthropometric measurements (weight and height) and body composition (BMI and body fat percentage) were performed for each subject in the laboratory. The subjects’ fat percentages were measured with bio-electrical device (BIA) (South Korea). Maximum oxygen consumption (VO2max) was measured using a modified Bruce test (16).
The exercise protocol consisted of one session of aerobic activity. Before, immediately after, and 24 hours after the exercise, 5 mL blood samples were obtained from the capital veins of participants. First, sampling was done in the resting state at 8 am from all the participants in fasting mode to measure the plasma levels of OXA, insulin, glucose, and insulin resistance. After the first sampling, to measure the subjects’ responses to acute aerobic exercise, patients were asked to run on a treadmill h/p/cosmos made in Germany connected to gas analyzer with 60% of maximal oxygen consumption until their energy expenditure reached to 300 calories (the least energy to control body weight per session). This amount was calculated from the gas analyzer data using exchange doxygen and carbon dioxide, through the following formula for each sample (17).
Energy expenditure = 3.9 × VO2 (l/min) + 1.1 × VCO2 (l/min)
The exercise protocol terminated after consuming 300 kcal energy as a result of acute activity by the participants. Immediately and after 24 hours in the fasting state, 5 mL blood was taken and was sent to the laboratory to measure the plasma levels of OXA, insulin, glucose and insulin resistance. Blood samples were withdrawn into heparinized tubes containing anticoagulant citrate or EDTA 5% and gently shaken to prevent clotting. Heparinized whole blood samples were immediately centrifuged at 200 to 300 rpm in 4°C for 20 minutes. Immediately after isolation, plasma samples were stored at -80°C until measuring the variables. Glucose concentration was measured using the enzymatic colorimetric method (glucose oxidase, Pars Test, Tehran, Iran) and the Auto Analyzer Selectra 2. Insulin was measured by enzyme-linked immunosorbent assay (ELISA) using Monobind kits. OXA was also measured by ELISA using Phoenix Pharmaceutical Inc. kits. Insulin resistance was calculated using the following homeostatic model assessment-insulin resistance (HOMA-IR) formula (18).

3.1. Statistical Analysis
Statistical analysis with respect to default parametric tests was performed using analysis of variance with repeated measures and Bonferroni post-hoc tests to evaluate the changes before, immediately after, and 24 hours after. These statistical tests were performed using statistical package for social science (SPSS) 22.0 for windows. A significant change was accepted at P ≤ 0.05.
4. Results
Anthropometric, physiological, and physical composition of the participants are shown in Table 1. The plasma levels of insulin and insulin resistance before, immediately after, and 24 hours after the aerobic exercise in control and experimental groups are presented in Table 2. There were no significant change in the levels of insulin and insulin resistance before, immediately after, and 24 hours after acute aerobic exercise in the two studied groups.
| Variables | Groups | |
|---|---|---|
| Control | Experimental | |
| Age, y | 46.60 ± 5.42 | 44.20 ± 3.70 |
| Height, cm | 174.98 ± 6.39 | 179.00 ± 5.03 |
| Weight, kg | 79.30 ± 6.35 | 82.53 ± 5.47 |
| Fat, % | 25.82 ± 3.82 | 23.27 ± 3.34 |
| BMI, kg/m2 | 26.02 ± 2.02 | 24.81 ± 2.76 |
| Waist-hip ratio, m | 0.96 ± 1.12 | 0.94 ± 2.33 |
| VO2max,mL/kg/min | 35.09 ± 4.07 | 37.09 ± 3.03 |
Anthropometric, Physiological, and Physical Composition of the Participantsa
| Variables | Sampling Time | ||
|---|---|---|---|
| Before | Immediately After | After 24 Hours | |
| Insulin, Mu/l | |||
| Control group | 13.72 ± 0.81 | 13.90 ± 0.77 | 13.61 ± 0.80 |
| Experimental group | 10.46 ± 2.25 | 10.28 ± 1.24 | 10.78 ± 2.21 |
| Insulin resistance | |||
| Control group | 5.64 ± 0.66 | 5.73 ± 0.84 | 5.11 ± 1.44 |
| Experimental group | 4.26 ± 1.06 | 3.59 ± 0.57 | 4.21 ± 1.48 |
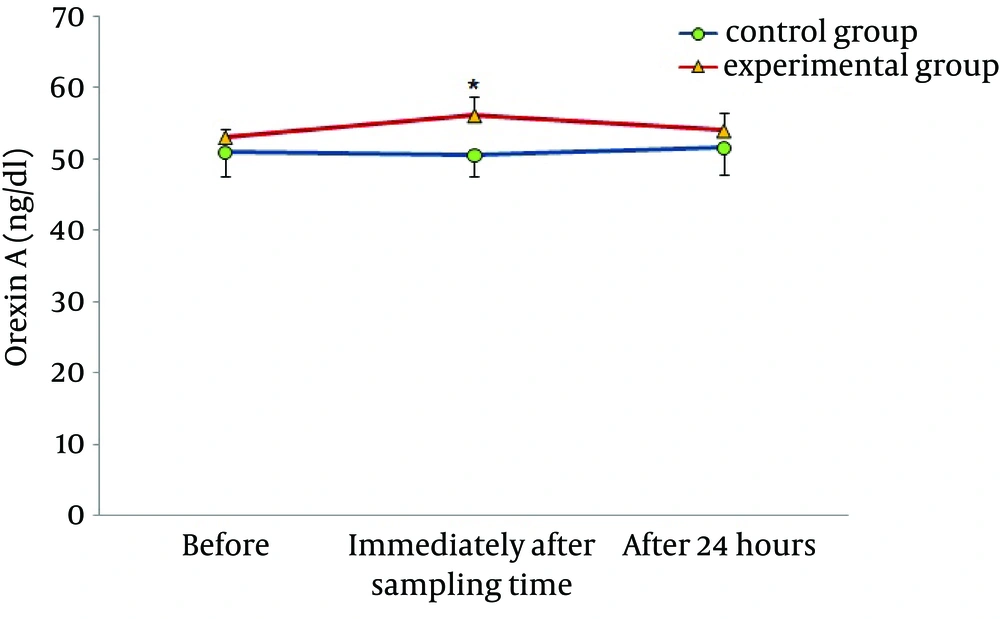
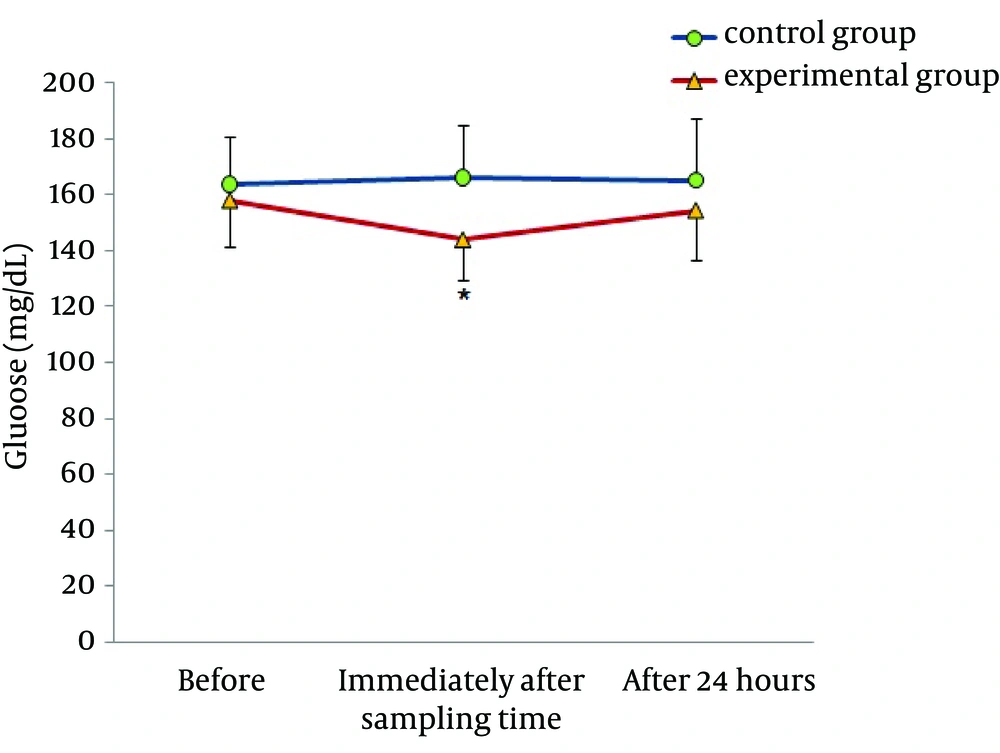
Figures 1 and 2 demonstrate the plasma levels of OXA and glucose in control and experimental groups, before, immediately after, and 24 hours after acute aerobic exercise. In the control group, there were no significant differences before and after the exercise. However, in the experimental group, significantly higher levels of OXA and lower levels of glucose were found immediately after, as compared with before the aerobic exercise. In both groups, there were no significant changes in these two indexes 24 hours after the aerobic exercise.
5. Discussion
In the current study, we observed an increase in plasma levels of OXA after acute aerobic exercise in males with type 2 diabetes. There are a few studies that investigated the effects of exercise in human specimens, but there are many studies in animal specimens with conflicting results. Martins et al. examined the effect of exercise on orexin levels in cerebrospinal fluid of 3-month-old male Wistar rats. They found that forced swimming inside a 45-cm-deep tank, with the water temperature set at 33°C for 30 minutes, with a load of 5% of body weight attached to the tail, increased the levels of OXA (9). In addition, Espana et al. reported that one week of wheel running activity in orexin-deficient rats alternatively eliminated the orexin deficiency complications (19). It seemed that increased orexin levels after physical activity was due to the increased motor activities (14). In addition, other factors such as increased oxygen consumption, heart rate, blood pressure, and body temperature, resulting in the activation of the sympathetic nervous system, could increase the orexin level (20, 21). Wu et al. (22) reported that treadmill exercise at speeds of 30, 50, and 105 m/min for 30 minutes did not change the brain levels of orexin in Doberman dogs. Inconsistent results obtained in animal models may be due to different training protocols or chosen subjects (22). A research by Messina et al. (15) was the first study conducted on human samples. They investigated the association between sympathetic nervous system activity and increased plasma levels of OXA. In agreement with our findings, they found an increase in plasma levels of OXA followed by 15 minutes cycling at 75 W and noted that increase in orexin was due to increased sympathetic activity and the increase in heart rate occurred along with the increase in orexin secretion, indicating the body sympathetic activity. On the other hand, some studies showed that brain injection of orexin could lead to increased activity of the sympathetic nervous system and increase the activity of motor nerves toward the heart (15). Studies also showed that orexin neurons had a major role in regulating the heart rates of mice and orexin administration increased heart rate and blood pressure (23). These findings show that the cerebral hypocretinergic pathway regulates the sympathetic nervous system with possible relationship of cause-effect between OXA and sympathetic activation. In addition, it is possible that some sympathetic responses are resulted from increased levels of orexin in the brain, because the brain OXA easily enters the blood stream by diffusion and affects the heart function, heart rate, and blood pressure (20).
Tsuneki et al. suggested that orexin-expressing neurons effectively response to nutritional conditions and increase the expression of orexin mRNA precursors in the condition of hunger and low blood sugar caused by insulin in lateral hypothalamus of mice (10). In agreement with this study, our findings showed coordinated changes in OXA and plasma levels of glucose, so that increase in OXA was associated with reduction in blood glucose. Researchers reported that stimulation of OXA secretion and its role in increased desire to take food is because of reduction in glucose levels. Therefore, increased secretion of OXA in this study may be due to low blood glucose resulted from physical activity (24, 25). Recent studies indicated that OXA increased glucose uptake by elevated translocation of the glucose transporter GLUT4 from cytoplasm into the plasma membrane (25). Hence, the effects of OXA and glucose are in interaction, so that increase in OXA may cause an increase in GLUT4 activity and decrease in blood glucose, and high blood sugar may decrease the expression of OXA in brain. The importance of this issue is due to the role of OXA in maintaining the hypothalamic sensitivity of insulin (26).Our results also showed an insignificant reduction in insulin levels and insulin resistance. These findings are inconsistent with the results of Jamurtas et al. (27). In their investigation, nine obese males were subjected to one session of aerobic exercise for 45 minutes at approximately 65% of maximal oxygen uptake. They observed that insulin resistance index dropped and insulin concentration decreased immediately after the activity (27). In agreement with our findings, Rodnick et al. reported that an aerobic exercise at 60% - 70% of VO2max for 30 - 60 minutes had no effect on glucose tolerance test in subjects with type 2 insulin-resistant diabetic (28). It seems that factors such as intensity, duration of activity, and changes in energy homeostasis as a result of aerobic activity are the main factors causing conflicting results in these studies (29). Probably, insufficient energy expenditure was the main reason of unchanged plasma levels of insulin and insulin resistance in this study. In our research, we tried to calculate the energy expenditure using respiratory gas analyzer and with consideration of the risks that may develop due to heavy activity for our non-athletic diabetic participants, the energy expenditure was 300 Kcal. That apparently was not enough to make a negative balance in these subjects and it made no changes in plasma levels of insulin and insulin resistance after the aerobic activity.
Since the possibility of metabolic disorders such as type 2 diabetes is high in people with low orexin secretion (8), the hope is that increase in OXA after physical activity in long term affects the effective factors on type 2 diabetes and insulin resistance. Our research is one of the few studies that evaluate the effect of acute aerobic exercise on changes in OXA in human samples and showing the significant effect of acute physical activity on OXA and glucose homeostasis. However, the interaction with insulin and insulin resistance was statistically insignificant. It seems that more negative energy balance than that of this research is necessary for the accurate study of these changes.