1. Background
Contamination of aquatic systems by heavy metal ions is one of the main concerns of environmentalists because of their high toxicity and accumulative properties within biological systems (1, 2). Mercury, as one of the most toxic metals in the environment, can be released from various sources such as batteries, paper and pulp, paint manufacturing and petrochemical industries. The maximum allowable concentration of total mercury specified as low as 5 μgL-1 for waste water drains, by the European Union (3). In the recent years, increasing pollution by mercury in Iran has been reported by some researchers (4-8). Thus, the necessity to reduce the amount of mercury ions in wastewater effluents of industries and stringent environmental safety regulations demand the removal of this heavy metal from various discharges to avoid contamination of the biological ecosystem. To date, many methods have been developed to remove mercury from aqueous solutions, such as coagulation (9), ultrafiltration (10) photo-catalytic degradation (11), ion exchange (12), and adsorption (7, 13, 14).
Among all these removal methods, adsorption has been regarded as the most promising technique for the removal of mercury from wastewater effluents due to its ease of operation, high efficiency, adsorption rate and the availability of a wide range of sorbents. Although, numerous adsorbent materials such as activated carbons (15), clay, minerals (16), zeolite, fibers (17, 18), bio-sorbents (19-21), resins (1) and nano-scale materials (22, 23) have been used, search for novel adsorbents for mercury ion removal from wastewaters has been a challenge and constituted in an important field of contemporary research (24).
Nowadays, many environmental chemists focused their research on using biopolymers as efficient adsorbents for separation and removal of heavy metal ions because of their easy availability, low cost, presence of a variety of functionalities and their non-toxic nature. Among various available biopolymers, chitosan, a type of harmless polysaccharide, is prepared by de-acetylation of natural chitin, a major component of crustacean shells and fungal biomass and it is readily available from seafood processing wastes. Chitosan has been widely used for removing many heavy metal ions from waste waters through complexation with the primary amine groups at C-2 position of glucosamine residues (25-27). However, some drawbacks of this biopolymer such as its softness, tendency to form gels in aqueous solution and unavailability of reactive binding sites in gel form limit its application. On the other hand, micron-sized chitosan-based adsorbents need large internal porosities to ensure adequate surface area for adsorption and diffusion limitation within the particles decreased adsorption rate and available capacity. In contrast, nano-sized supports possess quite good performance due to high specific surface area and absence of internal diffusion resistance. However, these nanomaterials could not be separated easily from the aqueous solution by filtration or centrifugation. Using magnetic nano-adsorbents to solve separation problems has received considerable attention in the recent years. These Magnetic nano-adsorbents can be easily collected by an external magnetic field and hence facilitate phase separation (28, 29). Iron oxide nanoparticles are the most common magnetic sorbents used in removal of pollutants because of their better magnetic properties, lower toxicity, and lower price. Among these nanoparticles, magnetite (Fe3O4) plays important role in different areas of chemistry, physics and materials sciences (25). To overcome the limitations and operational problems in application of unmodified chitosan and to exploit the magnetite nanoparticles advantages, chitosan coated magnetite nano-composite is proposed. A limited amount of published data is available regarding preparation, characterization and adsorption properties of nano-sized chitosan-magnetite composite and its application for the removal of heavy metals from waste waters (25, 27, 30-36).
2. Objectives
Petrochemical industry waste water, probably, is one of the major sources of ecosystem contamination in Khuzestan Province. Our objectives in this study, following our previous work on the preparation of chitosan coated nanoparticles (37), were to (I) evaluate the capability and behavior of one-step in-situ synthesized (MCNs) for the removal of mercury ions from complex matrixes of petrochemical waste water, (II) determine the optimum conditions for mercury removal in waste water matrix, and (III) assess the effect of batch and column modes on removal efficiency.
3. Materials and Methods
3.1. Chemicals and Solutions
Chitosan (600 - 1200 cp and 96% degree of deacetylation) was purchased from Primex (Iceland). A stock solution of Hg (II) (1000 mgL-1) was purchased from Merck (Darmstadt, Germany) and aqueous solutions of mercury ions at various concentrations were prepared using successive dilutions of the stock solution. FeCl2.4H2O, FeCl3.6H2O, ammonia solution (25%), glacial acetic acid, hydrochloric acid and sodium hydroxide were of analytical grade and obtained from Merck (Darmstadt, Germany). The petrochemical waste water was obtained from chlor-alkali unit of Mahshahr petrochemical industry with initial mercury ion concentration of 5.63 mgL-1.
3.2. Measurements
The Fourier transform infrared (FT-IR) spectra were scanned in the region of 400 - 4000 cm-1 in KBr pellets on Bruker (Tensor 27) FT-IR spectrometer. The dimensions and surface morphology of the MCNs were observed by means of a TESCAN (Mira-eXMU) field emission scanning electron microscope (FESEM). The concentrations of Hg (II) were determined by Chemtech Analytical Instrument model CTA-3000 (Bedford, England) Atomic Absorption Spectrometer via Cold Vapor technique (CV-AAS). Metrohm model 632 (Herisau, Switzerland) pH meter for pH measurements and a super-magnet (1.2 T, 10 cm × 5 cm × 2 cm) were used.
3.3. Preparation of Magnetic Chitosan Nanoparticles
Preparation of MCNs has been described previously (37). Briefly, chitosan (1% w/v) in acetic acid and ammonium hydroxide solutions (1 molL-1) were added drop-by-drop in a mixture of ferrous and ferric chlorides solutions while stirring vigorously. The prepared nanoparticles were collected using an external magnetic field and thoroughly washed by deionized water.
3.4. Batch Adsorption Experiments
Batch experiments were performed to investigate Hg (II) adsorption property on the prepared MCNs by placing 10 - 100 mg of nanoparticles in a series of flasks containing 50 mL of the petrochemical waste water at pH 4 - 8. Then the contents of the flasks were stirred in a stirrer at 15 - 40°C for 1 - 120 minutes with 100 - 750 rpm stirring. The residual concentration of the mercury ions in the waste water was determined by CV-AAS. The amount of Hg (II) adsorbed per gram of the MCNs was calculated according to the following equation:

Where qe (mgg−1) is the adsorption capacity, C0 and Ce (mgL−1) are the initial and equilibrium concentrations of mercury ions, respectively. V (L) is the volume of the solution and m (g) is the mass of dry adsorbent used. The percentage of Hg (II) removal was calculated using the following expression:

Where C0 and Ce are the initial and equilibrium concentrations of Hg (II) in waste water (mgL-1), respectively. The experimental variables studied for the extent of adsorption were pH, amount of adsorbent, contact time, stirring speed and temperature in the batch mode.
3.5. Column Adsorption Experiments
Column experiments were performed in a mini column (0.5 cm/10 cm) under optimum conditions obtained from the batch operations (section 3.4). For this purpose, 50 mg MCNs was packed in the column. A 50 mL volume of waste water under the optimum pH 6 at 20°C, passed continuously through the stationary bed of sorbent. The outgoing Hg (II) concentration was determined as described above.
4. Results
4.1. Characterization of Magnetic Chitosan Nanoparticles
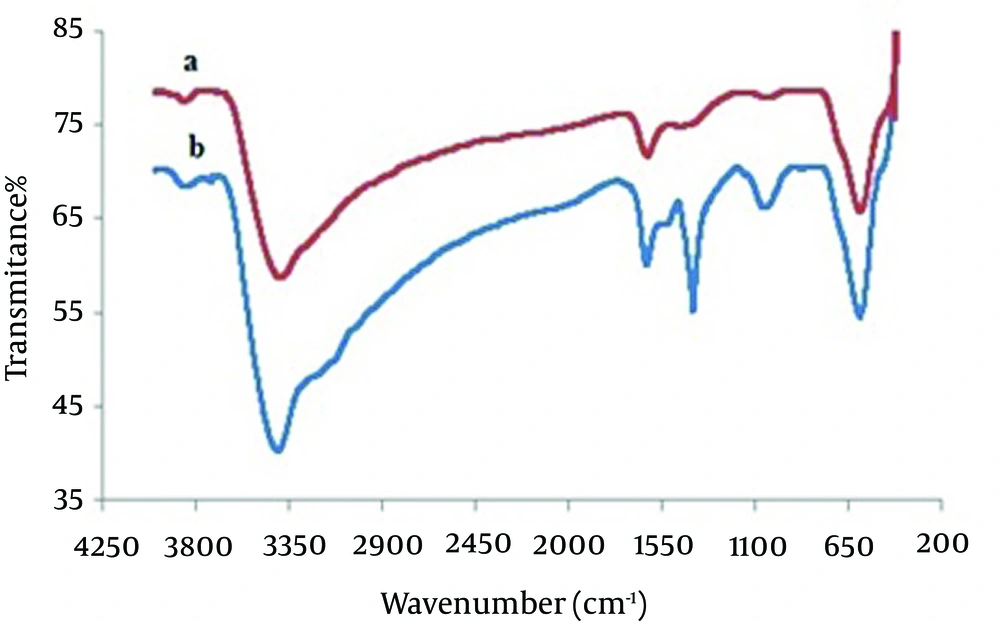
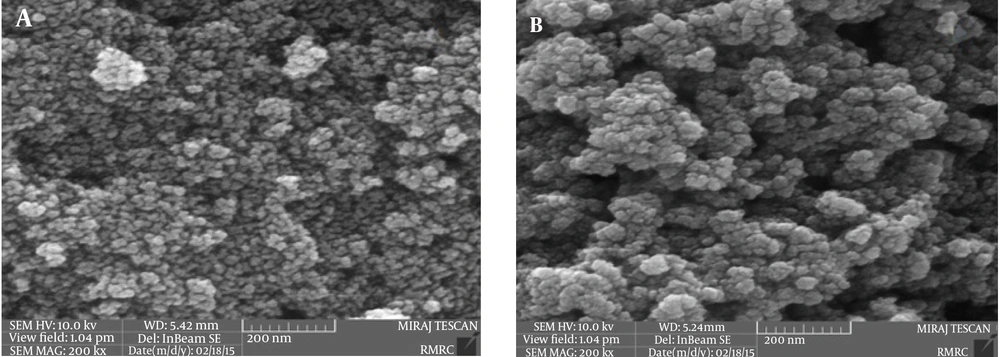
The structure of MCNs was studied by FT-IR spectroscopy. Figure 1A shows the FT-IR spectra of magnetite nanoparticles with major bands at 3392 and 598 cm-1 related to the O-H stretching vibrations of the adsorbed water on the magnetite nanoparticles and the characteristic peak of Fe3O4, respectively. As seen in Figure 1B, the adsorption band at 3406 cm-1 is assigned to the N-H and O-H stretching vibrations. The peaks at 1627 and 1403 cm-1 are caused by the N-H bending vibration and C-N stretching vibration, respectively (27, 28, 34, 35, 37). FESEM images for magnetite nanoparticles and MCNs are shown in Figure 2. As illustrated in Figure 2A, magnetite particles have a spherical shape with the diameter distribution of 10 - 20 nm. Figure 2B reveals that the MCNs sizes are slightly larger than those of magnetite particles (20 - 30 nm). Apparently, this observation suggests that magnetite nanoparticles were coated by chitosan biopolymer. However, the sizes of MCNs remain in the nanometer range.
4.2. Effect of pH
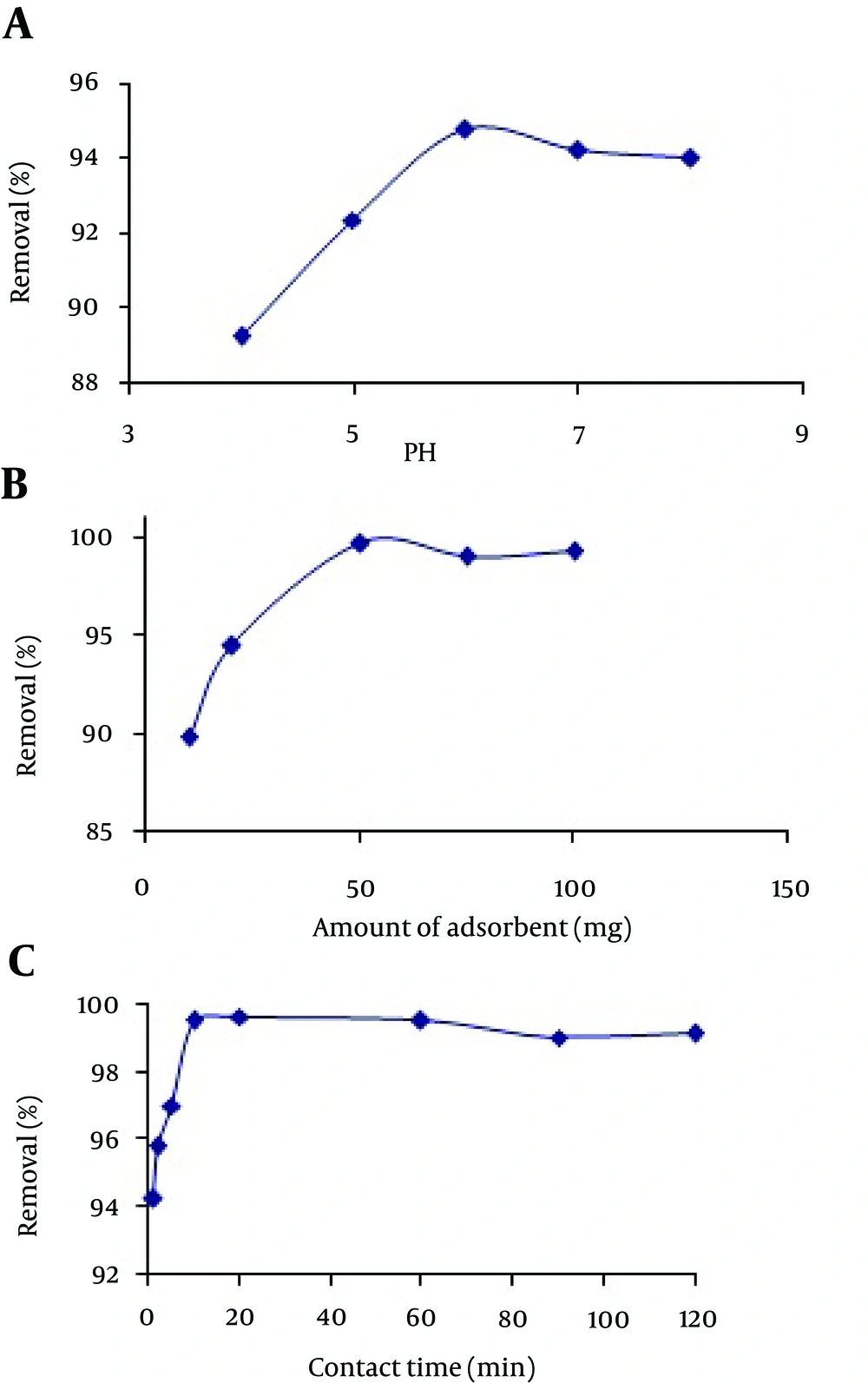
Initial pH of solution has serious impact on the adsorption process by affecting the surface properties of adsorbent and formation of various chemical forms of metal ions. To study the effect of pH on adsorption of Hg (II) on MCNs, a pH range of 4.0 - 8.0 was selected to avoid hydrolysis of metal ions in pHs above 8 and dissolution of MCNs in pHs lower than 3. The obtained results are presented in Figure 3A and it can be seen that the percentage of removal of Hg (II) on MCNs increased with the pH rising from 4 - 8 and reached to the maximum value of 94.8% at pH value of 6. Although, according to the results, the maximum value of removal was obtained at pH 6. In the pH values of 7 and 8, the adsorption efficiency was also favorable (94.0 - 94.2%). The results of previous studies indicated that a mixture of two mechanisms might be responsible for adsorption of Hg (II) by MCNs at pH value of 6. The presence of amino groups in chitosan (pKa = 6.5) helps it to adsorb transition metals via ion exchange and complex formation mechanisms. The abundance of H+ and Cl− ions in the medium may lead to ion exchange mechanism through the protonation of un-substituted amino groups, and the formation of the mercury complex anions (HgCl3−), as represented in the following equations:


On the other hand, the mechanism through which mercury ions adsorbed by the MCNs may be attributed to the complex formation between the metal ion and amine functional groups of the sorbent as shown below (18, 29, 34, 37):

4.3. Effect of Amount of Adsorbent
The effect of the amount of adsorbent as another important factor to obtain quantitative removal was investigated and optimized in the range of 10 - 100 mg of MCNs at pH 6. As can be seen in Figure 3 B, the maximum adsorption of Hg (II) was achieved when the amount of MCNs was 50 mg. Greater amounts of MCNs did not cause a considerable increase in the removal efficiency of mercury ions. Based on this result, an adsorbent amount of 50 mg was chosen as optimum value for further experiments.
4.4. Effect of Contact Time
Figure 3 C indicates the effect of contact time on the removal of Hg (II) ions from waste water by MCNs. It is apparent from this figure that the adsorption rate was high and reached equilibrium in 10 minutes with 99.8% removal percentage of Hg (II) ions. This rapid adsorption could be an evidence for the chemical binding or electrostatic forces between mercury ions and surface functional groups of MCNs. The adsorption of Hg (II) ions increased rapidly in the first 5 minutes and then slowed down until the equilibrium state was attained in 10 minutes. Afterwards, removal percentage remained almost constant. The lower time needed to reach the equilibrium state and rapid diffusion of metal ions in MCNs shows the great affinity of adsorbent to the adsorbate.
4.5. Effect of Temperature and Agitation Speed
The petrochemical waste water was agitated under optimum pH and amount of MCNs (50 mg) at different speeds (100 - 750 rpm) and temperature ranged from 15 - 40°C. Maximum adsorption efficiencies were achieved at 20°C and stirring speed of 500 rpm. The results showed that in almost all examined temperatures, the mercury ions adsorbed quantitatively and slowly reached the maximum value at 20°C, indicating that the temperature was not an effective factor in adsorption process in the examined range. In addition, stirring speeds higher than 500 rpm were not favorable and a tendency of Hg (II) ions to escape from the MCNs was observed. The lower percentage of removal at higher agitation speeds is probably due to the weakness of electrostatic forces between adsorbent and mercury ions at high speeds.
4.6. Kinetics of Adsorption
The results obtained from section 4.4 were exploited to further investigate the kinetic model that controls the adsorption process. Three kinetic models, pseudo-first-order (equation 6), pseudo-second-order (equation 7), and intra-particle diffusion (equation 8) were used to interpret the experimental data and verify the kinetic mechanism of adsorption and their equations are presented below (1, 29, 38):



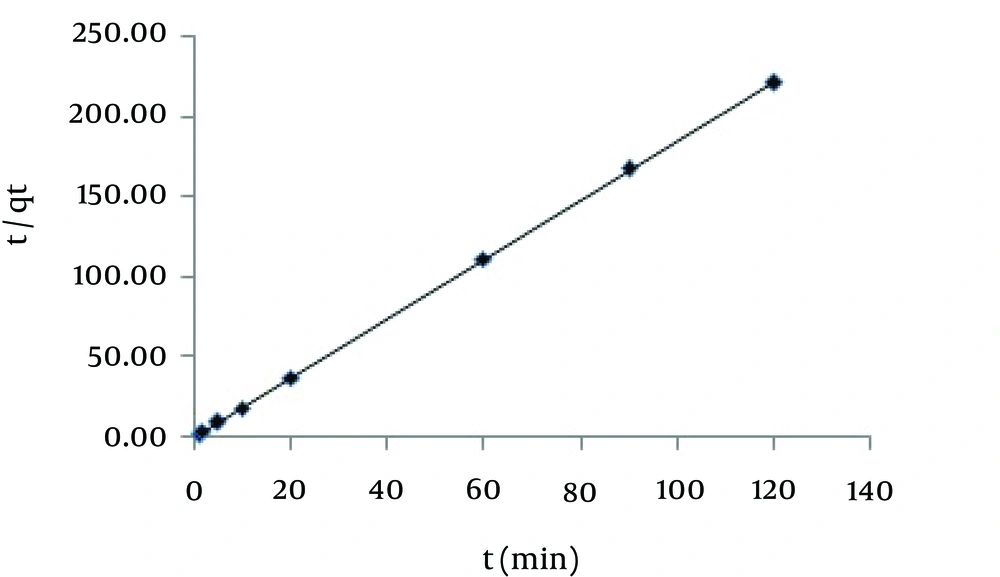
Where qe and qt are adsorption capacity at equilibrium and at different time intervals (mgg-1), respectively. K1, K2 and Kp are the rate constants of the pseudo-first-order (min−1), pseudo-second-order model (gmg-1min-1), and intra-particle diffusion (mgg-1min-0.5) models. The above-mentioned values can be determined from the slope and intercept of the lines which are plotted according to the equations 6 - 8. The obtained results showed that the pseudo-second-order model gives a better description for the experimental data. The kinetic parameters obtained from different models are represented in Table 1. The value of correlation coefficient for this plot, > 0.999, shows that the plot is of good linearity (Figure 4). Moreover, the equilibrium adsorption capacity calculated (qe,cal) based on pseudo-second-order model was much better in agreement with the experimental data (qe,exp), further demonstrating the kinetic adsorption processes follow pseudo-second-order model better than other kinetic models. This suggests that the rate-limiting step of Hg (II) ion onto MCNs is chemisorption, where valence forces are involved via electrons sharing between MCNs and Hg (II) ions or exchange between protonated MCNs and HgCl3- as mentioned in section 4.2 (29).
Kinetic Parameters for Hg (II) Ions Adsorption by Magnetic Chitosan Nanoparticles (MCNs)
4.7. Column Adsorption Mode
Table 2 shows that for three separate experiments in the column mode, the percent of mercury ions removal obtained was 81.2 - 85.0. The approximate time needed to perform the experiments, 30 minutes, compares unfavorably to the batch mode method. Moreover, a high efficiency of removal (> 99.8%) was observed in batch experiments.
5. Discussion
The present study focused on the removal of Hg (II) ions from complicated matrix of waste water produced at chlor-alkali section of the petrochemical industry using MCNs. However, many studies in removal of mercury ions via adsorption process were performed in spiked synthetic solutions (1, 2, 13, 17, 18) and few published research are available using adsorbents in real samples (23). In this work, optimization of various parameters affecting removal efficiency was performed in the real matrix of petrochemical waste water instead of synthetic waters to suppress the matrix effects. The results obtained from batch adsorption experiments showed that this synthesized adsorbent possesses an excellent ability to remove mercury ions from petrochemical waste water and complicated matrixes of the samples had no obvious effect on quantitative removal of these ions. The optimum conditions obtained for Hg (II) ions are easily available in real samples (pH 6 and solution temperature of 20°C). Very fast adsorption with an optimum contact time of 10 minutes and rapid settling of MCNs by an external magnetic field makes this adsorbent suitable for continuous flow water treatment systems. The proposed method was better than some previously reported research for fast kinetic and environmental safety of adsorbent (1, 13, 14). FT-IR analysis confirmed the presence of nitrogens in –N–H and C–N bonds contributed to the adsorption of Hg (II) ions. Kinetic studies showed that the pseudo-second order model is appropriate to describe the adsorption process.