1. Background
Phenol is a toxic chemical in the water and wastewater and can be considered one of the most important organic pollutants (1). Phenol is very stable in water (2) and is one of the common organic pollutants (3). Phenol and its derivatives can be found in operations and industries including petroleum refineries, gas and coke oven industries, pharmaceuticals, explosive manufacture, phenol-formaldehyde resin manufacture, plastic and varnish industries and related metallurgical operations and a number of other industries. Improper discharge of these materials can cause damage to the environment, especially water supplies (4). Health effects of phenol depend on the extent of absorption and duration of exposure and include mucosal irritation, burning, skin burns and systemic toxicity associated with hypotension, rapid heartbeat and coma (5). Based on world health organization (WHO) guidelines, phenol concentration in drinking water must not exceed 0.1 microgram per liter. These guidelines are based on taste and odor caused by combination of chlorine with phenol (6). Phenolic compounds in the environment have harmful effects such as reducing growth and resistance to disease and increasing mortality of aquatic organisms and growth of weeds. When they enter the groundwater, serious ecological problems will occur (7). According to what mentioned above, removing phenol from the environment, particularly from water and water resources, is important. There are various methods for treating phenol in the sewage including absorption on activated carbon, chemical oxidation, electrochemical oxidation and biodegradation methods (8). Among these methods, biological systems are more widely used because they have certain advantages compared to other methods. One of the major advantages of these methods is that they are more compatible with the environment. Another advantage of these methods compared with chemical methods is that they usually do not use any harmful chemicals to the environment. Therefore, the disposal of wastewater and sludge using these processes have less adverse effects on water resources compared to chemical processes (5). Despite the toxic properties of phenol, many microorganisms can be used phenol and its derivatives under aerobic conditions that will be open the path to economic development for the treatment of contaminated wastes (9). Many aerobic bacteria are capable to use aromatic compounds as the sole carbon and energy source. Although both aerobic and anaerobic microorganisms are able to degrade phenol, aerobic processes are preferred; because they grow faster than anaerobes and usually transform organic compounds to inorganic compounds. A typical pathway for metabolizing phenol is to hydroxylate the ring to form catechol and then to open the ring through the ortho- or meta-oxidation (10).
Biological treatment is a method that uses the potential of microorganisms to clean up contaminated environment (11). Effluents containing 300 - 400 milligrams per liter of phenol are suitable for biological treatment. Among microorganisms, bacteria are especially important in treating wastewater contaminated with phenol (12). Kotresha et al. in 2008, a sample of Pseudomonas aeruginosa was separate of waste an industrial zone, that had an ability of the analysis a phenol concentration of 1300 mg L-1, a period of time had 156 hours (13).
Also, a study conducted by Moussavi et al. in 2010 showed that granules biological were used in the reactor.
A sequencing batch reactor by adding phenol concentration of 2000 mg L-1is reduced removal efficiency of the system (14). Phenol and phenolic compounds degradation is done mainly by P. aeruginosa and other microorganisms such as Phanerochaete chrysosporium, Rhodococcus spp, Candida tropicalis, Basillus brevise, Penicillium chrysogenum and Alcaligenes (12).
The phenol-degrading yeasts include Trichosporon cutaneum, Phanerochaete chrysosporium, and Pleurotus ostreatus. The phenol-degrading fungi include Candida tropicalis and Fusarium flucciferium (7).
2. Objectives
In this study, to evaluate the ability of phenol removal by P. aeruginosa, we used media containing salts and various concentrations of phenol. Salt was applied to speed up phenol degradation.
3. Methods
The P. aeruginosa strain used in this study had been isolated from petroleum-contaminated soil (15). To evaluate the toxicity of phenol and salt, different concentrations of these two compounds were added to flasks containing mineral medium. Then, P. aeruginosa bacterium was cultured in the nutrient broth medium. After 24 hours, the temperature was set at 35°C and the optical absorbance was measured using a spectrophotometer at a wavelength of 600 nm (16).
This is an experimental study conducted as a pilot in a batch reactor with different concentrations of phenol (25, 50, 100, 150, 300 and 600 mg L-1) and sodium (0, 5, 10, 25, and 50%) during 9, 12 and 15 hours. for determination of the optimized experimental condition, an experimental design was used, and the one-factor-at-a-time method was also applied. During three days, from 5 experimental and 3 control samples, 18 samples were taken a day forming a sample size of 54 samples for each phenol concentration. Given the number of phenol concentrations (n = 6), a total of 324 samples were analyzed using a spectrophotometer at a wavelength of 600 nm.
3.1. Culture and Adaptation of Bacteria
The composition of the mineral medium is contained (in gL-1): K2HPO4 0.8, KH2PO4 0.2, CaSO4•2H2O 0.05, MgSO4 H2O 0.5, FeSO4•7H2O 0.09, (NH4)2SO4 (15). To prepare the solid mineral medium, we added 2 g agar to each 100 mL. Then, the medium was autoclaved up to the boiling point and poured into the plate after cooling. After cooling, 50 mL of phenol solution (at a concentration of 50 mg L-1) was added to the plate surface (11). A colony of bacteria was taken using a sterile loop and cultured on plates. After 20 minutes, the plates were inverted and incubated for 2 days at 35°C. In these conditions, the growth of bacteria in the media containing phenol was investigated. It was done for compliance of bacteria with phenol and to maintain good growth conditions.
3.2. Preparation of Bacterial Inoculum
Fifty milliliter of the nutrient broth medium was prepared. Two flasks were used and 20 mL of the nutrient broth culture medium was added to each. The remaining 10 mL was poured into the third flask. A small magnet was placed inside each flask and their lids were closed with cotton with aluminum foils stretched over them. The medium was autoclaved at 121°C for 15 minutes and cooled to ambient temperature. A colony of bacteria grown in the medium was taken using a sterile loop and added into the nutrient broth culture media (the 20-mL media). The cotton was put back on the lid and the flask placed into an incubator shaker. The temperature was set at 35°C and the samples were placed for 24 hours at this temperature. After this period, the growth rates of bacteria were measured using a spectrophotometer (DR 5000) at 600 nm (16).
3.3. Evaluation of Bacteria Growth Power
This study aimed to assess the growth of bacteria in the mineral medium with various concentrations of phenol and salt. In the experimental design, a total of 30 test conditions, all of which being replicated three times, were considered. The amount of bacteria added to each flask was equal to five percent (1.5 mL) of bacteria with an optical density of 1. Each flask contained 30 mL of mineral medium closed with cotton after sterilizing and dropping a small magnet inside. The amounts of phenol and salt needed (based on the experimental design) were added to each flask and two flasks were used as the control.
The amount of bacteria (inoculum) obtained in the previous section was added to each flask (except control) (10). The flasks were placed in an incubator shaker for 72 hours at 35°C and the optical density of all samples (and control 1) was measured at a wavelength of 600 nm during 0, 9, 12, 15, 24, 48 and 72 hours.
3.4. Counting the Colonies
Given that in the turbidity test (measuring the optical density) no distinction can be considered between the live and dead bacteria, the plate count method was used to count the colonies. After the nutrient agar medium was prepared, it was autoclaved at 121°C for 15 minutes and then poured into plates after cooling. For each test flask, 2 plates were used. After cooling and hardening of the nutrient agar media, the contents of test flasks with different concentrations were cultured on the plates. Then, 100 mL of the obtained concentrations was added to the nutrient agar medium and uniformly distributed over the surface of the plate by a Pasteur pipette. Each samples had at least two replications. Finally, the number of colonies was counted after the plates were incubated for 24 hours at 35°C.
4. Results
So far, various methods have been used to remove phenolic compounds, but the use of bacteria is the most commonly used and the cheapest method for this purpose.
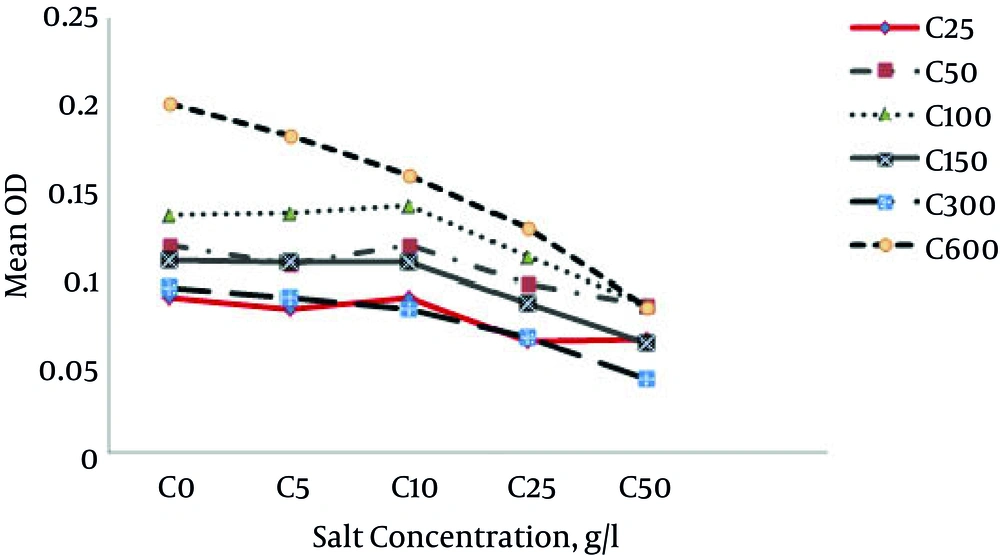
In the present study, at phenol concentration of 25 mg L-1, the growth rate of bacteria changed when the salt increased. At zero salt conditions, the mean growth rate of bacteria was zero. At salt concentration of 1% and above, the mean growth rate reduced.
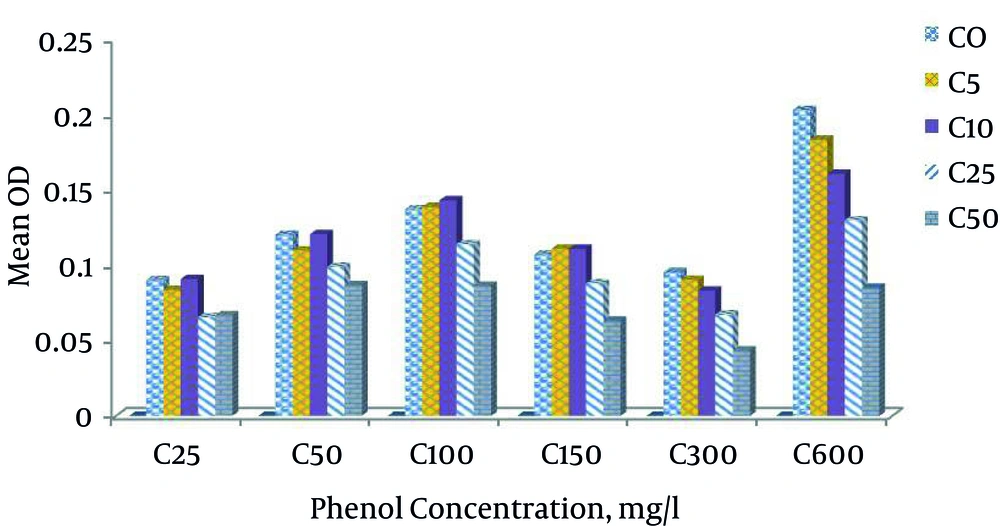
At phenol concentration of 50 mg L-1, by adding 0%, 0.5%, 1%, 2.5% and 5%, the bacteria growth means were 0.1201, 0.109, 0.1205, 0.0988 and 0.0866, respectively. At the phenol concentration of 100 mg and salt concentrations of 0%, 0.5%, 1%, 2.5% and 5%, bacteria growth rates were 0.137, 0.138, 0.143, 0.113 and 0.086, respectively. As expected, it increased up to a salt concentration of 1%. The highest growth was at salt concentration of 1%. Afterwards, a decrease was observed and at phenol concentration of 150 mg L-1, the bacteria growth rates at salt percentages of 0, 0.5, 1, 2.5 and 5% were 0.106, 0.1108, 0.1107, 0.087 and 0.063, respectively. The highest bacteria growth was at the salt concentration of 5 and at phenol concentration of 300 mg L-1 and at salt percentages of 0%, 0.5%, 1%, 2.5% and 5%, the mean bacterial growth rates were 0.095, 0.090, 0.083, 0.067, and 0.043, respectively. The growth rate decreased with an increase in salt. The growth was the highest when salt concentration was zero. At phenol concentration of 600, when there was no salt, the growth rate was the highest, but the addition of salt reduced the growth rate. According to Figure 1, the highest mean growth was at phenol concentration of 600 mg L-1. Minimal growth occurred at the phenol concentration of 25 mg L-1. Generally, in all phenol concentrations, bacteria growth rates were reduced at salt concentrations of 1% and above (Figures 1 and 2).
Phenol acts as a toxin to P. aeruginosa bacterium at the concentration of 600 mg L-1, but acts as a carbon source for P. aeruginosa at a certain concentration.
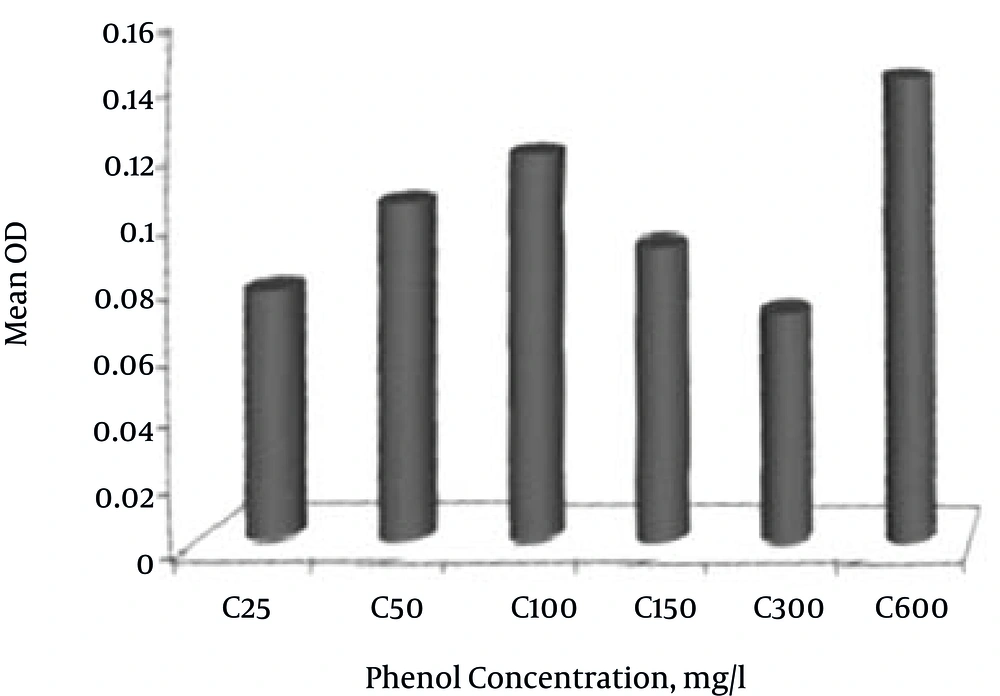
The results showed that with increasing phenol concentration to 600 mg L-1, the bacteria growth rate increased. The highest bacteria growth rate was at a phenol concentration of 600 mg L-1 (Figure 3).
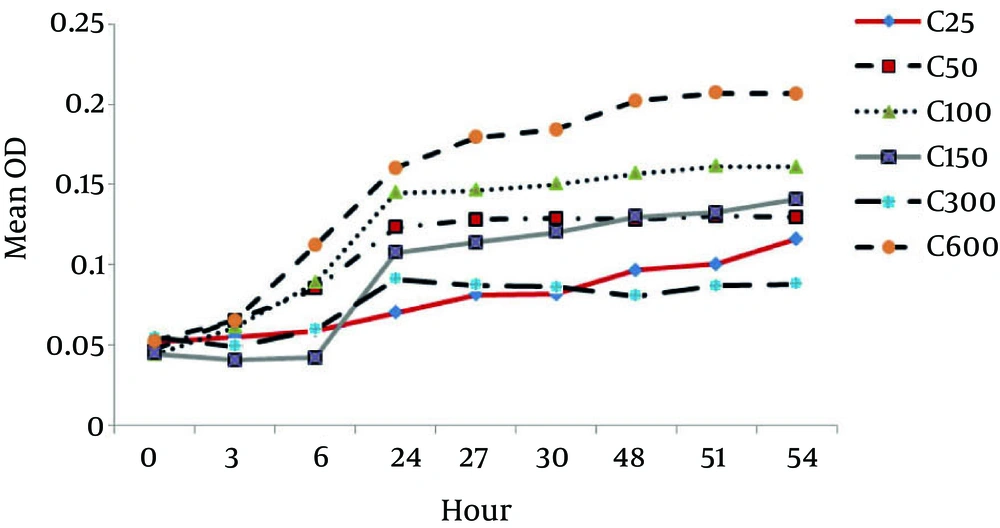
According to Figure 4, the growth rate of P. aeruginosa increased with time. This growth increased with increasing the phenol concentration up to 600 mg L-1and the phenol acted as a source of carbon and energy. However, after a concentration of 600 mg L-1, the growth rate reduced. With increasing phenol, the growth reduced and became a toxin to P. aeruginosa bacteria.
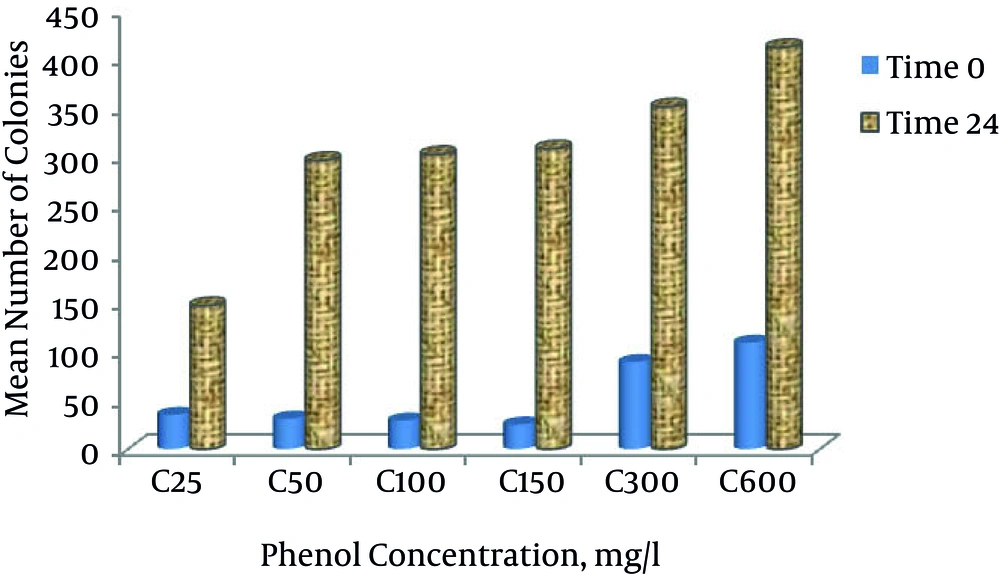
Based on Figure 5, the number of colonies at different phenol concentrations at time zero was 24. According to this count, the number of colonies at the phenol concentration of 600 mg L-1 at time zero was the highest. According to Figure 5, at time zero and 24, with increasing phenol concentration, the growth and the number of colonies increased.
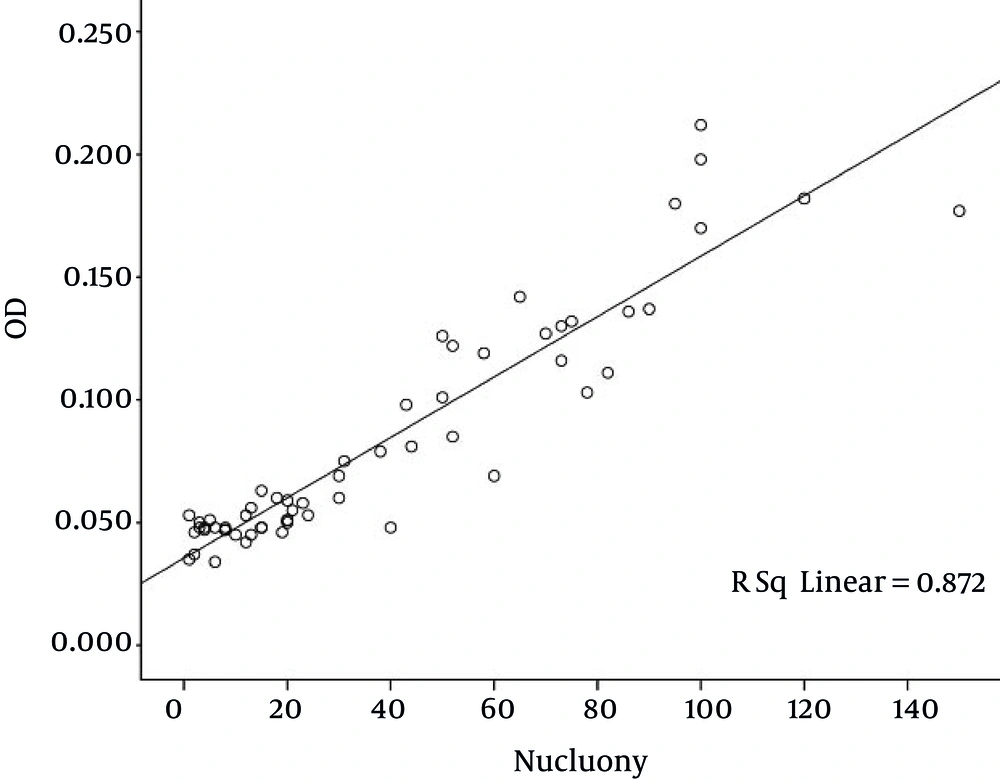
Based on Figure 6, there was a strong relationship between the colonies and the optical density (OD). With an increase in growth of P. aeruginosa bacteria, the number of colonies reduced and, as mentioned, by increasing the phenol concentration the bacteria growth increased. The bacteria growth and the number of colonies had an inverse relationship. However, in any case the number of colonies was greater than the first time.
5. Discussion
A study by Eskandari et al. in 2009 entitled “investigation of the growth and biodegradation of phenol by bacteria isolated from industrial effluents in laboratory conditions” used a domestic separation mechanism for phenol-contaminated wastewater in the Esfahan steel company (ESCO). They tried to adapt an isolate and ultimately remove phenol by the isolate. After a lag phase of 24 and 48 hours, the isolate grew and the amount of phenol after 264 and 288 hours reached zero milligrams per liter. It was identified to be a Gram-negative coccobacillus possibly from P. aeruginosa species (17). Koutny et al. (2003) isolated P. aeruginosa strains as phenol-degrading materials (18). Whiteley et al. (2001) showed that the isolates in bioreactors could change features like physiology, versatility and performance of the phenol-degrading P. aeruginosa in industrial bioreactors (19). In 2007, Afzal et al. conducted an analytical study on the characteristics of phenol removal in medium containing salts by bacteria species of P. aeruginosa and Pseudomonas pseudomallei. They found that both species of bacteria were very useful in removal of phenol and the ability of P. aeruginosa to remove phenol was higher than other species. In the presence of various salts (Nacl, Kcl, Na2SO4, K2SO4), the aeruginosa species could remove phenol approximately 1.53 and 1.34 times faster than the previous species. Pseudomonas aeruginosa could grow at a high phenol concentration (1500 mg L-1) (20). Watanabe et al. (1999) found that phenol concentrations higher than 400 - 500 mg in batch culture of activated sludge bacteria had a negative effect on the population of degrading bacteria and reduced bacteria growth (21).
A study by Shourian et al. concluded that the higher concentration of 700 mg per liter of growth, a potentially slow destruction and also an increase above 1000 mg phenol after 85 hours resulted in complete elimination of phenol (22).
A study by Arutchelvan et al. on phenol degradation using Bacillus brevis at 0 to 144 hours at concentrations of 750 to 1750 mg per liter was carried out, concluding that higher concentrations of phenol longer takes completely destroyed (23).
In a study conducted by Arutchelvan et al. on the two Pseudomonas cepacia and Bacillus brevis at different times and different concentrations of the species cepacia, initial concentrations of 1000, 1500, 2000 and 2500 mg of phenol per hour to 48 , 96, 108 and 144 hours of removal and destruction of 97.7%, 99.3%, 98.7% and 98.6% reached (4).
The two species of organisms were very effective in destruction of phenol and phenol; the lag phase increased with increasing concentration. Pseudomonas cepacia and Bacillus brevis in 2500 and 1750 mg phenol concentrations were decreased in 144 hours (4).
According to previous studies and comparison with the present study, the growth of bacteria strains was studied for 2 weeks and the bacteria growth measured. During the study, it was concluded that the bacteria can grow at a salt concentration of 1%, which had the highest growth rate. At higher concentrations, the bacteria growth rate reduced. The bacteria acted as toxin at phenol concentration 600 mg L-1, but at a certain concentration, it acted as a carbon source for Pseudomonas aeruginosa. Increasing the phenol concentration increased the bacteria growth so that the highest bacteria growth rate was at a concentration of 600 mg L-1 phenol. This strain was only able to analyze the concentration of 600 mg L-1 phenol and they had low and negligible growth for concentrations above this level during the first 24 hours of incubation. However, as time passed, the strain could adapt to high concentrations and degrade phenol. However, at very high phenol concentrations, the bacteria acted as a toxin. This study showed that to achieve effective biological phenol degradation, phenol concentration higher than 600 mg L-1 should not be used in effluent ponds, because this concentration has negative effects on the growth of phenol-degrading bacteria. Pseudomonas aeruginosa has the highest ability to degrade organic pollutants including phenolic substances. These Gram-negative and rod-shaped bacteria do not act in fermentation. The high ability of P. aeruginosa to analyze organic materials is not only because they can produce catabolic enzymes, but also because they have the ability to regulate metabolic pathways (24).
Increasing phenol concentration in the media increases resistance in the present bacteria. During the growth, if the bacteria do not have a carbon source except phenol, they will use phenol as the sole carbon source in the medium. Using this resistant bacterium and creating optimum conditions for biological treatment, we can treat the wastewater contaminated with the carbonated compound and thus reduce costs and reduce environmental problems caused by the remains of this hazardous substance.