1. Background
Leishmaniasis, a significant and often underestimated tropical infectious disease, is caused by obligate intracellular protozoan organisms of the Leishmania genus. Transmission typically occurs through the bites of mosquitoes belonging to the Phlebotomus genus (1-3). Leishmaniasis is endemic in over 98 countries, affecting an estimated 350 million people worldwide, with an additional 2 to 2.5 million new cases reported annually (4, 5).
Clinical manifestations of leishmaniasis are classified into three forms cutaneous, mucosal, and visceral depending on the Leishmania species involved (3, 6). Approximately 75% of cutaneous Leishmaniasis (CL) cases are concentrated in ten countries: Afghanistan, Algeria, Brazil, Colombia, Costa Rica, Ethiopia, Iran, Sudan, Peru, and Syria (4, 7). In Iran, the estimated incidence of CL is around 20,000 cases across various regions, although the actual number is likely higher (1, 6, 8-10).
The epidemiological and clinical characteristics of CL vary depending on interactions between different factors such as parasite strain, host, vector, and environmental and socioeconomic risk factors (3, 9, 11, 12). In Iran, CL manifests in two forms: Anthroponotic CL (ACL, urban or dry type) and zoonotic CL (ZCL, rural or wet type), caused by Leishmania tropica and Leishmania major, respectively (9, 13). Currently, 19 of Iran's 31 provinces are affected by ZCL (6).
The spread of CL is influenced by various factors, including environmental changes, agricultural developments, urban sprawl, migration to endemic areas, proximity of homes to rodent habitats, construction of water reservoirs like dams, and fluctuations in vector control efforts targeting CL (9, 10, 14). In Iran, approximately 80% of annual Leishmaniasis cases are attributed to ZCL, 0.5% to visceral Leishmaniasis, and the remainder to ACL (15). However, these figures likely underestimate the true incidence due to underreporting in underserved areas, challenges in disease diagnosis, and limitations in the sensitivity of laboratory diagnostics (16).
The economic impact of CL is significant, particularly in developing countries, where it places a considerable burden on families, communities, and nations as a whole (17). In Iran, the estimated economic burden of the disease is approximately 5 - 6 million dollars (18).
Khuzestan province, situated in southwestern Iran, holds strategic importance due to its environmental conditions and geographical location (19-22). In recent decades, the province has undergone significant climatic changes (23), which have become critical risk factors for the spread of vector-borne diseases like CL (24). Currently, Khuzestan is one of the most significant endemic areas for CL in Iran (25). Both ACL and ZCL have been reported in Khuzestan, with ZCL being considered endemic (6).
Numerous studies have been conducted on the epidemiology of CL in various regions of Khuzestan province in recent years. For example, between 2008 and 2013, health centers in ten cities within the province reported a total of 4,137 confirmed cases of CL through blood slide examinations, revealing the presence of the amastigote form of the *Leishmania* parasite (26). Due to its proximity to Iraq, a country with a high prevalence of CL, Khuzestan province experiences continuous cross-border movement throughout the year, further contributing to the spread of the disease (27).
Hoveyzeh county is recognized as an endemic area for CL (26). Despite the existing literature on CL epidemiology in various parts of Khuzestan province (26, 28-30), only one study specifically focused on Hoveyzeh county, authored by Jayrvnd and Vaziri (31).
2. Objectives
In light of the significant medical and economic impact of CL, as well as its zoonotic potential, it is essential to conduct epidemiological studies to determine the prevalence of the disease and implement a comprehensive program to prevent and control its spread. Given the lack of comprehensive studies on CL in Hoveyzeh county, this research aims to investigate the epidemiology of CL from 2016 to 2021 in this region.
3. Methods
3.1. Study Design and Area
The current study is a retrospective, cross-sectional, descriptive study based on available data. It was conducted in Hoveyzeh county, which is located at 31.46 degrees North and 48.07 degrees East, in the western part of Khuzestan province. The county comprises two cities: Rofayyeh and Hoveyzeh (Figure 1).
3.2. Setting
The statistical population includes all individuals who were treated and followed up in healthcare centers from 2016 to the end of 2021 in Hoveyzeh county, and were clinically diagnosed with CL, with confirmation through laboratory tests. The epidemiological data for these individuals are summarized in the study forms. All individuals diagnosed with CL during this five-year period were included in the analysis. Patient information is kept strictly confidential, and data is entered using codes rather than any form that could identify the patients.
After receiving ethical clearance from Jundishapur University of Medical Sciences, Ahvaz, the researchers referred to the Hoveyzeh Health Center, where patient information was registered. The required information for each patient, including age, sex, lesion type (dry or wet), place of residence, history of travel, number and shape of wounds, location of wounds, time of infection, type of treatment, and recurrence, was entered into the relevant checklist. All patient information remained confidential, with data being entered using codes instead of names.
3.3. Statistical Analysis
The data was analyzed using GraphPad Prism software version 15, employing descriptive statistical tests, including mean, standard deviation, frequency, percentages, and 95% confidence interval (CI).
4. Results
A total of 628 patients with CL were identified between 2016 and 2021. Of these, 324 (51.6%) were male and 304 (48.4%) were female. The mean age of the patients was 16.58 ± 15.17 years, with an age range of 1 month to 80 years. The age group with the highest proportion of cases (45.4%) was individuals between 0 and 10 years old, while the group with the lowest proportion of cases (3.7%) consisted of individuals over 50 years old.
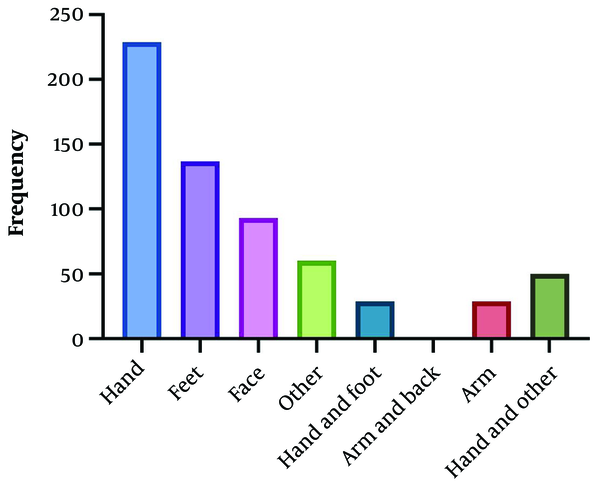
In terms of residency, 68.2% of the patients lived in urban areas, while 31.8% resided in rural areas. The largest occupational group was students, comprising 186 individuals (29.6%). Analysis of wound characteristics revealed that wet wounds were more prevalent (95.22%) than dry wounds. The number of wounds in patients ranged from one to 25, with an average diameter of 1.82 ± 1.6 cm. The distribution of affected limbs was as follows: 44.4% on the hand, 21.8% on the foot, 14.8% on the face, 4.6% on both the hand and foot, 4.8% on the arm, and 9.6% on other body parts.
A review of the medical records revealed that approximately 96.97% of patients had no history of previous scars. Regarding treatment, most cases received simultaneous local treatment with cryotherapy (46.82%) (Table 1 and Figure 2).
| Characters and Categories | No (%) |
|---|---|
| Gender | |
| Male | 324 (51.6) |
| Female | 304 (48.4) |
| Age group | |
| 0 - 10 | 285 (45.4) |
| 11 - 20 | 138 (22) |
| 21 - 30 | 105 (16.7) |
| 31 - 40 | 53 (8.4) |
| 41 - 50 | 24 (3.8) |
| > 50 | 23 (3.7) |
| Residence | |
| Urban | 428 (68.2) |
| Rural | 200 (31.8) |
| Job | |
| Child | 217 (34.6) |
| Student | 186 (29.6) |
| Homemaker | 120 (19.1) |
| Employee | 32 (5.1) |
| Soldier | 19 (3) |
| Farmer | 10 (1.6) |
| Other | 44 (7) |
| Travel history | |
| Yes | 43 (6.8) |
| No | 585 (93.2) |
| Treatment | |
| Topical glucantime & cryotherapy | 294 (46.8) |
| Topical glucantime | 205 (32.6) |
| Systemic glucantime | 121 (19.2) |
| Cryotherapy | 4 (0.7) |
| Other | 4 (0.7) |
Demographic and Clinical Characteristics of Cutaneous Leishmaniasis Patients in Hoveyzeh County, During 2016 - 2021
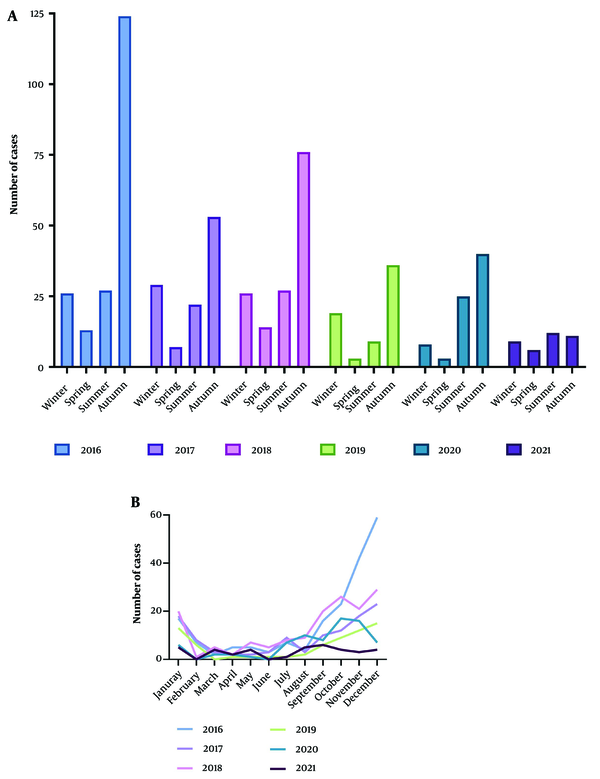
The incidence of CL showed a fluctuating pattern from 2016 to 2021. There was an upward trend from 2017 to 2018, followed by another increase from 2019 to 2020, with a subsequent decline from 2016 to 2021. The highest proportion of cases (54%) occurred during the fall season, with December recording the highest frequency (22%) among the months, followed by November and October. The peak year for incidence was 2016, which accounted for 30.3% of the cases (Figure 3).
5. Discussion
The present study was conducted to investigate the epidemiological and clinical features of CL in Hoveyzeh county, Khuzestan province, Iran. A total of 628 patients with CL were examined over a period of five years. Neglected tropical diseases often affect impoverished populations due to poor hygiene, substandard housing, and limited access to basic nutrition. Leishmaniasis, as a neglected tropical disease, is particularly prevalent in disadvantaged areas (32).
The prevalence of CL varies across different regions, with demographic factors influencing its distribution. The disease affects individuals of all age groups, though some regions show a higher prevalence among the elderly, while others, including this study, show a higher prevalence among the young. In this study, the age group of 1 - 10 years comprised the largest proportion of patients (45.4%), with 186 students (29.6%). These findings align with previous studies conducted in Saudi Arabia, Dasht-e-Azadegan, and Kerman (28, 33, 34). They indicate an initial increase in CL prevalence among individuals under 15 years of age, followed by a decline due to acquired immunity, making children and students more susceptible (33).
According to the results of this study, the incidence of CL was higher in men, which is consistent with the findings of the Hamedan and Dasht-e-Azadegan studies (28, 35). However, this result differs from that of the study by Akhavan et al. (36). Most studies demonstrate that both men and women are susceptible to infection, but men are more likely to be exposed due to occupational factors such as agricultural work, outdoor activities, and clothing choices that increase vector exposure during travel to endemic areas (35, 37).
Most cases were concentrated in urban areas, a finding that aligns with studies conducted in Kashan by Doroodgar and Doroodgar (38), and in Dasht-e-Azadegan by Kassiri et al. (28). However, this contrasts with the findings of Al-Waaly and Shubber (37) in Iraq. It is important to note that prevalence rates can vary between rural and urban populations in different cities. Urban residents often have easier access to health centers, which may lead to lower reporting of CL cases in rural areas. Additionally, inadequate environmental hygiene and lack of proper sewage systems, particularly in marginal urban areas, contribute to increased mosquito populations, resulting in higher reported cases of CL in cities (28).
The prevalence of lesions was 44.4% on the hands and 21.8% on the feet. Since the hands are often uncovered and easily exposed to bites from infected sand flies, lesions are more commonly found on the hands than on other parts of the body. These findings corroborate previous research conducted in Dezful and Neyshabur (30, 39). Wet wounds (95.22%) were more common than dry wounds, and 96.97% of patients had no history of previous scars. The mean wound diameter in this study was 1.82 ± 1.60 cm, with the number of lesions ranging from one to 25. The maximum number of lesions documented in a study conducted in Jahrom was 30. A high number of lesions can be attributed to several factors, including the characteristics and behavior of blood-sucking insects, the density of infected sand flies in the endemic area, and the number of bites per feeding by the sand fly (40).
The standard treatment in Iran typically includes the administration of meglumine antimoniate (glucantime) injections, cryotherapy, or a combination of both. Most patients in this study received simultaneous local treatment with cryotherapy (46.8%), which aligns with findings from Libya and Dasht-e-Azadegan (41) but differs from the Doroodgar et al.’s study (38). The seasonal peak of CL occurred in autumn, with the highest number of cases in December (21.97%). This could be due to the disease's incubation period, weather conditions, and the peak activity of sand flies during this time (38). These findings align with research conducted in Kashan (39) and Isfahan (38), but contrast with the higher winter prevalence reported in Kermanshah (42).
Several factors influence the location of lesions on the body, including the species and biting habits of sand flies, social and cultural activities, and weather conditions. Clothing style is one of the most significant factors in determining the location of lesions (17). In general, outdoor occupations increase the risk of CL due to greater exposure to sand fly bites (32).
One of the major challenges in controlling CL, particularly in urban areas, is the combined influence of multiple factors in disease transmission. Variations in the cause of the disease and the vector, the presence of human and animal reservoirs, environmental factors such as weather, agriculture, and rainfall patterns, as well as human behaviors, including habits, residential areas, and working conditions, all play critical roles in disease transmission and infection (43).
From 2016 to 2021, there was a decrease in the incidence of CL, which can be attributed to rising average temperatures that have reduced the occurrence of the disease. Specifically, climate change, warmer temperatures, and lower humidity levels effectively reduce the breeding and activity of sand flies. Sand flies prefer moist environments to lay eggs, survive, and complete their life cycle. Reduced moisture availability disrupts their life cycle, thereby impacting disease incidence. Community education, promotion of personal protective measures, and increased awareness of CL have been effective strategies in reducing the number of cases in recent years (44).
5.1. Limitations and Strengths of the Study
A limitation of this study was the lack of identification of the *Leishmania* parasite species through molecular studies. However, its strength lies in the comprehensive evaluation of the epidemiology of CL in Hoveyzeh county over several years.
5.2. Conclusions
The findings of this study revealed a decreasing trend in the incidence of CL in Hoveyzeh county; however, the disease remains endemic and a significant health concern in the region. To effectively control and prevent CL, educational initiatives are crucial. These programs should focus on raising awareness among residents about health practices, disease transmission, personal protective measures, early detection for successful treatment outcomes, and the use of insect repellents and insecticide-treated bed nets. Given the endemic nature of the area, implementing a rodent control program within a 500-meter radius around homes is advisable prior to initiating sand fly control efforts. In addition, regular inspections by local health authorities are recommended.